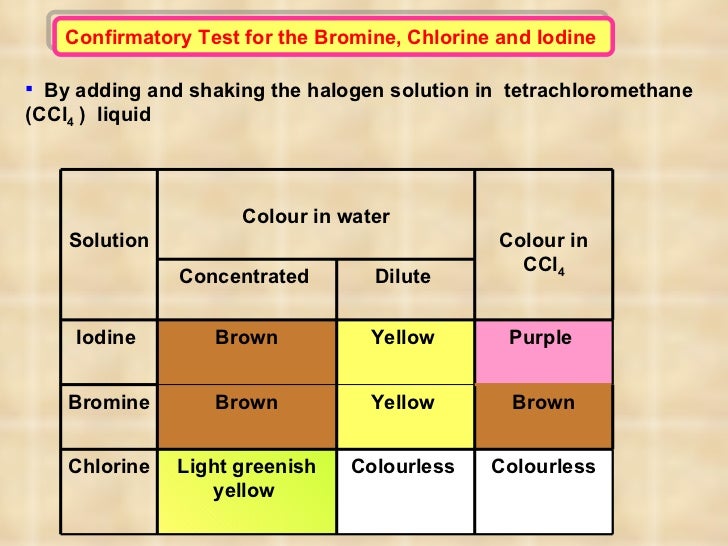
Chlorine Color Aqueous Solution . Transfer a drop of chlorine water onto the paper using a glass rod. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. Acidic and bleaching properties of halogen solutions. Place a piece of universal indicator paper on a white tile. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: At room temperature (20 °c), the physical state of the halogens changes as you go down the group. When chlorine is added to an aqueous iodide solution, a reaction takes place: Chlorine + potassium iodide → potassium chloride + iodine. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. A solution of chlorine can displace iodine from potassium iodide solution: The transition metals form colored ions, complexes, and compounds in aqueous solution. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine.
from www.slideshare.net
Chlorine + potassium iodide → potassium chloride + iodine. Transfer a drop of chlorine water onto the paper using a glass rod. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: A solution of chlorine can displace iodine from potassium iodide solution: When chlorine is added to an aqueous iodide solution, a reaction takes place: Place a piece of universal indicator paper on a white tile. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The transition metals form colored ions, complexes, and compounds in aqueous solution. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +.
Oxidation & reduction
Chlorine Color Aqueous Solution When chlorine is added to an aqueous iodide solution, a reaction takes place: Chlorine + potassium iodide → potassium chloride + iodine. A solution of chlorine can displace iodine from potassium iodide solution: At room temperature (20 °c), the physical state of the halogens changes as you go down the group. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Place a piece of universal indicator paper on a white tile. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. Acidic and bleaching properties of halogen solutions. When chlorine is added to an aqueous iodide solution, a reaction takes place: Transfer a drop of chlorine water onto the paper using a glass rod. The transition metals form colored ions, complexes, and compounds in aqueous solution. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +.
From woelen.homescience.net
Science made alive Chemistry/Solutions Chlorine Color Aqueous Solution A solution of chlorine can displace iodine from potassium iodide solution: Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: The transition metals form colored ions, complexes, and compounds in aqueous solution. Acidic and bleaching properties of halogen solutions. At room. Chlorine Color Aqueous Solution.
From www.chegg.com
Solved 3.3 Experiment 2 Aqueous chlorine and aqueous Chlorine Color Aqueous Solution Chlorine + potassium iodide → potassium chloride + iodine. Place a piece of universal indicator paper on a white tile. Acidic and bleaching properties of halogen solutions. A solution of chlorine can displace iodine from potassium iodide solution: Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. When chlorine is added to an aqueous. Chlorine Color Aqueous Solution.
From www.slideserve.com
PPT Transition Metal Chemistry and Coordination Compounds PowerPoint Chlorine Color Aqueous Solution Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. Chlorine + potassium iodide → potassium chloride + iodine. Acidic and bleaching properties of halogen solutions. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Transfer a drop of chlorine water. Chlorine Color Aqueous Solution.
From www.indiamart.com
Chlorine Solution at Rs 5/kg 7782505, तरल क्लोरीन Davis Chemical Chlorine Color Aqueous Solution A solution of chlorine can displace iodine from potassium iodide solution: Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: The transition metals form colored ions, complexes, and compounds in aqueous solution. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. When chlorine is added to. Chlorine Color Aqueous Solution.
From edu.rsc.org
Halogens in aqueous solution and their displacement reactions Chlorine Color Aqueous Solution Transfer a drop of chlorine water onto the paper using a glass rod. Chlorine + potassium iodide → potassium chloride + iodine. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. Place a piece of universal indicator paper on a white tile. Acidic and bleaching properties of halogen solutions. When chlorine (as a gas. Chlorine Color Aqueous Solution.
From brainly.in
cupric chloride witch colour Brainly.in Chlorine Color Aqueous Solution A solution of chlorine can displace iodine from potassium iodide solution: Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. When chlorine is added to an aqueous iodide solution, a reaction takes. Chlorine Color Aqueous Solution.
From www.youtube.com
Ferric Chloride Color, Solubility in Water FeCl3 Ion(III) chloride Chlorine Color Aqueous Solution The transition metals form colored ions, complexes, and compounds in aqueous solution. Chlorine + potassium iodide → potassium chloride + iodine. When chlorine is added to an aqueous iodide solution, a reaction takes place: Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. Place a piece of universal indicator paper on a white tile.. Chlorine Color Aqueous Solution.
From www.carolina.com
Calcium Chloride Solution, 1.0 M Aqueous, Laboratory Grade, 500 mL Chlorine Color Aqueous Solution Place a piece of universal indicator paper on a white tile. A solution of chlorine can displace iodine from potassium iodide solution: When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. When chlorine is added to an aqueous iodide solution, a reaction takes place: Transfer a. Chlorine Color Aqueous Solution.
From www.fishersci.com
Aluminum Chloride, 0.1 M Solution, Spectrum Chemical, Quantity 1 L Chlorine Color Aqueous Solution Acidic and bleaching properties of halogen solutions. Place a piece of universal indicator paper on a white tile. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. Chlorine + potassium iodide → potassium chloride + iodine. Transfer a drop of chlorine water onto the paper using a glass rod. Chlorine will displace bromine or iodine. Chlorine Color Aqueous Solution.
From www.chemedx.org
Demonstrating the Colors of Transition Metal Complex Ions Chemical Chlorine Color Aqueous Solution Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. Place a piece of universal indicator paper on a white tile. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: A solution of chlorine can displace. Chlorine Color Aqueous Solution.
From fphoto.photoshelter.com
precipitate silver chloride chemistry reaction Fundamental Chlorine Color Aqueous Solution Chlorine + potassium iodide → potassium chloride + iodine. Transfer a drop of chlorine water onto the paper using a glass rod. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Acidic. Chlorine Color Aqueous Solution.
From www.slideshare.net
Other Chem Presentation )) Chlorine Color Aqueous Solution When chlorine is added to an aqueous iodide solution, a reaction takes place: Acidic and bleaching properties of halogen solutions. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Chlorine + potassium iodide → potassium chloride + iodine. The transition metals form colored ions, complexes, and. Chlorine Color Aqueous Solution.
From www.alamy.com
Chlorine displacing iodine. Chlorine gas bubbling through an aqueous Chlorine Color Aqueous Solution Transfer a drop of chlorine water onto the paper using a glass rod. Place a piece of universal indicator paper on a white tile. When chlorine is added to an aqueous iodide solution, a reaction takes place: Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. When chlorine (as a gas or dissolved in. Chlorine Color Aqueous Solution.
From www.youtube.com
How to make a Stannous Chloride Solution (Tin (II) Chloride) YouTube Chlorine Color Aqueous Solution Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. Place a piece of universal indicator paper on a white tile. Transfer a drop of chlorine water onto the paper using a glass rod. A solution of chlorine can displace iodine from potassium iodide solution: The transition metals form colored ions, complexes, and compounds in aqueous. Chlorine Color Aqueous Solution.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Chlorine Color Aqueous Solution Transfer a drop of chlorine water onto the paper using a glass rod. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The transition metals form colored ions, complexes, and compounds in aqueous solution. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. Chlorine will displace bromine or iodine. Chlorine Color Aqueous Solution.
From chemistry.com.pk
Colours of Transition Metal Ions in Aqueous Solution [Infographic Chlorine Color Aqueous Solution Chlorine + potassium iodide → potassium chloride + iodine. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The transition metals form colored ions, complexes, and compounds in aqueous solution. Place a piece of universal indicator paper on a white tile. When chlorine is added to an aqueous iodide solution, a reaction takes place: Acidic. Chlorine Color Aqueous Solution.
From www.chegg.com
In an aqueous chloride solution cobalt(II) exists in Chlorine Color Aqueous Solution A solution of chlorine can displace iodine from potassium iodide solution: Transfer a drop of chlorine water onto the paper using a glass rod. Chlorine + potassium iodide → potassium chloride + iodine. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Acidic and bleaching properties of halogen solutions. Place a piece of universal indicator. Chlorine Color Aqueous Solution.
From www.youtube.com
Chlorine gas is passed in an aqueous potassium iodide solution to form Chlorine Color Aqueous Solution Place a piece of universal indicator paper on a white tile. When chlorine is added to an aqueous iodide solution, a reaction takes place: A solution of chlorine can displace iodine from potassium iodide solution: Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br. Chlorine Color Aqueous Solution.
From www.sciencemadness.org
Sciencemadness Discussion Board FeCl2 Powered by XMB 1.9.11 (Debug Chlorine Color Aqueous Solution Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. A solution of chlorine can displace iodine from potassium iodide solution: Acidic and bleaching properties of halogen solutions. Transfer a drop of chlorine water onto the paper using a glass rod.. Chlorine Color Aqueous Solution.
From fineartamerica.com
Iron Chlorides Photograph by Martyn F. Chillmaid/science Photo Library Chlorine Color Aqueous Solution When chlorine is added to an aqueous iodide solution, a reaction takes place: Place a piece of universal indicator paper on a white tile. Acidic and bleaching properties of halogen solutions. Transfer a drop of chlorine water onto the paper using a glass rod. Chlorine + potassium iodide → potassium chloride + iodine. Cl 2 (aq) + 2kbr (aq) →. Chlorine Color Aqueous Solution.
From www.youtube.com
Bromine Water + Sodium Iodide YouTube Chlorine Color Aqueous Solution Acidic and bleaching properties of halogen solutions. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. A solution of chlorine can displace iodine from potassium iodide solution: When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Transfer a drop of. Chlorine Color Aqueous Solution.
From www.youtube.com
How to make Stannous Chloride Solution SNCL2 (Tin (II) Chloride) .Easy Chlorine Color Aqueous Solution When chlorine is added to an aqueous iodide solution, a reaction takes place: Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. Chlorine + potassium iodide → potassium chloride + iodine. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. Fluorine and chlorine are gases,. Chlorine Color Aqueous Solution.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Chlorine Color Aqueous Solution At room temperature (20 °c), the physical state of the halogens changes as you go down the group. Transfer a drop of chlorine water onto the paper using a glass rod. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Fluorine and chlorine are gases, bromine. Chlorine Color Aqueous Solution.
From www.youtube.com
Chlorine Water + Sodium Iodide YouTube Chlorine Color Aqueous Solution At room temperature (20 °c), the physical state of the halogens changes as you go down the group. Transfer a drop of chlorine water onto the paper using a glass rod. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Chlorine + potassium iodide → potassium chloride + iodine. Fluorine and chlorine are gases, bromine. Chlorine Color Aqueous Solution.
From www.onlinemathlearning.com
Chemical Reactions IGCSE Chemistry (solutions, examples, worksheets Chlorine Color Aqueous Solution Acidic and bleaching properties of halogen solutions. A solution of chlorine can displace iodine from potassium iodide solution: Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. Chlorine + potassium iodide → potassium chloride + iodine. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes. Chlorine Color Aqueous Solution.
From sciencenotes.org
Transition Metal Ion Colors Chlorine Color Aqueous Solution A solution of chlorine can displace iodine from potassium iodide solution: When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. When chlorine is added to an aqueous iodide solution, a reaction. Chlorine Color Aqueous Solution.
From fphoto.photoshelter.com
science chemistry precipitation reaction silver chloride sodium Chlorine Color Aqueous Solution Transfer a drop of chlorine water onto the paper using a glass rod. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. A solution of chlorine can displace iodine from potassium iodide solution: Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: When chlorine (as a gas or dissolved. Chlorine Color Aqueous Solution.
From www.slideshare.net
Oxidation & reduction Chlorine Color Aqueous Solution Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. Place a piece of universal indicator paper on a white tile. When chlorine is added to an aqueous iodide solution, a reaction takes place: Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: At room temperature (20 °c), the physical state. Chlorine Color Aqueous Solution.
From fphoto.photoshelter.com
science chemistry compound cupric chloride Fundamental Photographs Chlorine Color Aqueous Solution Acidic and bleaching properties of halogen solutions. The transition metals form colored ions, complexes, and compounds in aqueous solution. Place a piece of universal indicator paper on a white tile. Transfer a drop of chlorine water onto the paper using a glass rod. When chlorine is added to an aqueous iodide solution, a reaction takes place: A solution of chlorine. Chlorine Color Aqueous Solution.
From www.thestudentroom.co.uk
Chemistry question OCR The Student Room Chlorine Color Aqueous Solution A solution of chlorine can displace iodine from potassium iodide solution: Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. Transfer a drop of chlorine water onto the paper using a glass rod. Chlorine + potassium iodide → potassium chloride + iodine. At room temperature (20 °c), the physical state of the halogens changes as. Chlorine Color Aqueous Solution.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Chlorine Color Aqueous Solution Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. Transfer a drop of chlorine water onto the paper using a glass rod. Acidic and bleaching properties of halogen solutions. A solution of chlorine can displace iodine from potassium iodide solution: When chlorine (as a gas or dissolved in water) is added to sodium bromide solution,. Chlorine Color Aqueous Solution.
From www.reddit.com
Precipitate Colors of Metal Ions in Aqueous Ammonia and Sodium Chlorine Color Aqueous Solution Chlorine + potassium iodide → potassium chloride + iodine. The transition metals form colored ions, complexes, and compounds in aqueous solution. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. At room temperature (20 °c), the physical state of the. Chlorine Color Aqueous Solution.
From www.sciencephoto.com
Chlorine displacing iodine Stock Image A500/0386 Science Photo Chlorine Color Aqueous Solution When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Transfer a drop of chlorine water onto the paper using a glass rod. Acidic and bleaching properties of halogen solutions. A solution of. Chlorine Color Aqueous Solution.
From www.slideshare.net
Redox Chlorine Color Aqueous Solution When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Acidic and bleaching properties of halogen solutions. Transfer a drop of chlorine water onto the paper using a glass rod. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2 (aq) chlorine +. A solution. Chlorine Color Aqueous Solution.
From tanjimscientific.com
Benzalkonium Chloride Aqueous Solution Extra Pure Tanjim Scientific Chlorine Color Aqueous Solution Chlorine + potassium iodide → potassium chloride + iodine. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. When chlorine is added to an aqueous iodide solution, a reaction takes place: Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The. Chlorine Color Aqueous Solution.