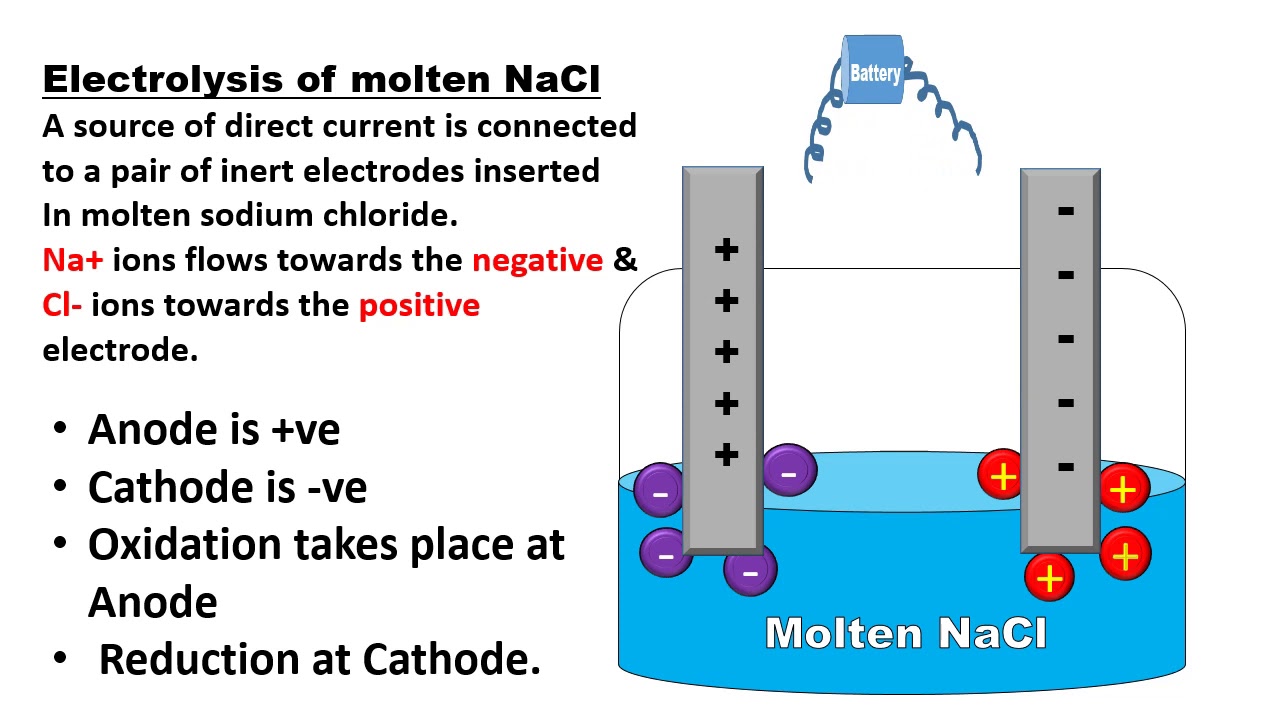
Why We Use Electrodes In Electrolysis . In an electrolytic cell, however, the. Reactive metals are extracted from their ores using electrolysis. We will look at three. Ionic compounds conduct electricity when molten or in solution. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Reactive metals are extracted from their ores using electrolysis. A chemical change takes place when atoms and ions lose or gain electrons. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Ionic compounds conduct electricity when molten or in solution. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their ores using electrolysis. Electrolysis, process by which electric current is passed through a substance to effect a chemical change.
from madisonmeowmercado.blogspot.com
A chemical change takes place when atoms and ions lose or gain electrons. Ionic compounds conduct electricity when molten or in solution. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Reactive metals are extracted from their ores using electrolysis. Reactive metals are extracted from their ores using electrolysis. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. In an electrolytic cell, however, the. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Ionic compounds conduct electricity when molten or in solution. Ionic compounds conduct electricity when molten or in solution.
Anode and Cathode in Electrolysis
Why We Use Electrodes In Electrolysis Reactive metals are extracted from their ores using electrolysis. A chemical change takes place when atoms and ions lose or gain electrons. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Ionic compounds conduct electricity when molten or in solution. Ionic compounds conduct electricity when molten or in solution. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Reactive metals are extracted from their ores using electrolysis. In an electrolytic cell, however, the. Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their ores using electrolysis. Reactive metals are extracted from their ores using electrolysis. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. We will look at three.
From byjus.com
Why is a DC used in electrolysis? Why We Use Electrodes In Electrolysis Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their ores using electrolysis. In an electrolytic cell, however, the. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Electrolysis, process by. Why We Use Electrodes In Electrolysis.
From blog.thepipingmart.com
Why are carbon electrodes used in electrolysis? Why We Use Electrodes In Electrolysis Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Ionic compounds conduct electricity when molten or in solution. Ionic compounds conduct electricity when molten or in solution. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in. Why We Use Electrodes In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Why We Use Electrodes In Electrolysis A chemical change takes place when atoms and ions lose or gain electrons. Reactive metals are extracted from their ores using electrolysis. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in. Why We Use Electrodes In Electrolysis.
From study.com
Electrolysis of Aqueous Solutions Lesson Why We Use Electrodes In Electrolysis A chemical change takes place when atoms and ions lose or gain electrons. Ionic compounds conduct electricity when molten or in solution. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Reactive metals are extracted from their ores using electrolysis. We will look at three. In an electrolytic cell, however, the. Think of. Why We Use Electrodes In Electrolysis.
From www.alamy.com
Electrolysis of copper sulphate solution using copper electrodes Stock Why We Use Electrodes In Electrolysis Ionic compounds conduct electricity when molten or in solution. Ionic compounds conduct electricity when molten or in solution. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. In this chapter, we have described various galvanic cells in which a spontaneous. Why We Use Electrodes In Electrolysis.
From courses.lumenlearning.com
Electrolysis General Chemistry Why We Use Electrodes In Electrolysis Ionic compounds conduct electricity when molten or in solution. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. In an electrolytic cell, however, the. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction. Why We Use Electrodes In Electrolysis.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at Why We Use Electrodes In Electrolysis Ionic compounds conduct electricity when molten or in solution. Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their ores using electrolysis. We will look at three. In an electrolytic cell, however, the. Reactive metals are extracted from their ores using electrolysis. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting. Why We Use Electrodes In Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID4966899 Why We Use Electrodes In Electrolysis Ionic compounds conduct electricity when molten or in solution. Ionic compounds conduct electricity when molten or in solution. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Reactive metals are extracted from their ores using electrolysis. A chemical change takes place when atoms and ions lose or gain. Why We Use Electrodes In Electrolysis.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Why We Use Electrodes In Electrolysis Reactive metals are extracted from their ores using electrolysis. A chemical change takes place when atoms and ions lose or gain electrons. We will look at three. In an electrolytic cell, however, the. Ionic compounds conduct electricity when molten or in solution. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Reactive metals are extracted from their. Why We Use Electrodes In Electrolysis.
From mungfali.com
Electrolysis Process Diagram Why We Use Electrodes In Electrolysis Reactive metals are extracted from their ores using electrolysis. Reactive metals are extracted from their ores using electrolysis. In an electrolytic cell, however, the. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Electrolysis, process by which electric current is passed through a substance to effect a chemical. Why We Use Electrodes In Electrolysis.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte Why We Use Electrodes In Electrolysis We will look at three. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Reactive metals are extracted from their ores using electrolysis. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution.. Why We Use Electrodes In Electrolysis.
From www.chemistrylearner.com
Analytical Chemistry Chemistry Learner Why We Use Electrodes In Electrolysis A chemical change takes place when atoms and ions lose or gain electrons. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. We will look at three. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In this chapter, we have described various galvanic cells. Why We Use Electrodes In Electrolysis.
From www.slideserve.com
PPT 17.78 Electrolysis & Applications PowerPoint Presentation ID Why We Use Electrodes In Electrolysis Reactive metals are extracted from their ores using electrolysis. Reactive metals are extracted from their ores using electrolysis. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. We will look at three. Reactive metals are extracted from their ores using electrolysis.. Why We Use Electrodes In Electrolysis.
From hadassah-has-friedman.blogspot.com
Anode and Cathode in Electrolysis HadassahhasFriedman Why We Use Electrodes In Electrolysis In an electrolytic cell, however, the. Ionic compounds conduct electricity when molten or in solution. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Reactive metals are extracted from their ores using electrolysis. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In this chapter,. Why We Use Electrodes In Electrolysis.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Why We Use Electrodes In Electrolysis Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. A chemical change takes place when atoms and ions lose or gain electrons. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Reactive metals are extracted from their ores using electrolysis. In this chapter, we have described various galvanic. Why We Use Electrodes In Electrolysis.
From en.ppt-online.org
Electrolysis online presentation Why We Use Electrodes In Electrolysis In an electrolytic cell, however, the. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Ionic compounds conduct electricity when molten or in solution. We will look at three. Electrolysis, process by which electric current is passed through a substance. Why We Use Electrodes In Electrolysis.
From quizunbestowed.z21.web.core.windows.net
Why Are Inert Electrodes Used In Electrolysis Why We Use Electrodes In Electrolysis Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Reactive metals are extracted from their ores using electrolysis. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. A chemical change takes place when atoms and ions lose or gain electrons. We will look at three. Ionic compounds conduct electricity. Why We Use Electrodes In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation ID297961 Why We Use Electrodes In Electrolysis We will look at three. In an electrolytic cell, however, the. Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Think of electrolysis and electrolytic. Why We Use Electrodes In Electrolysis.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS Why We Use Electrodes In Electrolysis Reactive metals are extracted from their ores using electrolysis. We will look at three. Ionic compounds conduct electricity when molten or in solution. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Ionic compounds conduct electricity. Why We Use Electrodes In Electrolysis.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Why We Use Electrodes In Electrolysis We will look at three. Ionic compounds conduct electricity when molten or in solution. In an electrolytic cell, however, the. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Ionic compounds. Why We Use Electrodes In Electrolysis.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students Why We Use Electrodes In Electrolysis In an electrolytic cell, however, the. We will look at three. Ionic compounds conduct electricity when molten or in solution. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Reactive metals are. Why We Use Electrodes In Electrolysis.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Why We Use Electrodes In Electrolysis Ionic compounds conduct electricity when molten or in solution. In an electrolytic cell, however, the. Reactive metals are extracted from their ores using electrolysis. We will look at three. Reactive metals are extracted from their ores using electrolysis. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. The. Why We Use Electrodes In Electrolysis.
From courses.lumenlearning.com
Electrolysis Chemistry Why We Use Electrodes In Electrolysis Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their ores using electrolysis. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in. Why We Use Electrodes In Electrolysis.
From brainly.in
electrolysis of acidified water using platinum electrodes gives Why We Use Electrodes In Electrolysis Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Reactive metals are extracted from their ores using electrolysis. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. We will look at three. Reactive metals are extracted from their ores using electrolysis. A chemical change takes place when atoms and ions. Why We Use Electrodes In Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Why We Use Electrodes In Electrolysis In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Reactive metals are extracted from their ores using electrolysis. In an electrolytic cell, however, the. Reactive metals are extracted from their ores using electrolysis. Electrolysis, process by which electric current is passed through a substance to effect a chemical. Why We Use Electrodes In Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Why We Use Electrodes In Electrolysis Reactive metals are extracted from their ores using electrolysis. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Ionic compounds conduct electricity when molten or in solution. Ionic compounds conduct electricity. Why We Use Electrodes In Electrolysis.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Why We Use Electrodes In Electrolysis Ionic compounds conduct electricity when molten or in solution. A chemical change takes place when atoms and ions lose or gain electrons. In an electrolytic cell, however, the. Reactive metals are extracted from their ores using electrolysis. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. We will look at three. Ionic. Why We Use Electrodes In Electrolysis.
From study.com
Faraday's Laws of Electrolysis Definition & Equation Video & Lesson Why We Use Electrodes In Electrolysis A chemical change takes place when atoms and ions lose or gain electrons. We will look at three. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Ionic compounds conduct electricity when molten or in solution. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell.. Why We Use Electrodes In Electrolysis.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Why We Use Electrodes In Electrolysis In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Reactive metals are extracted from their ores using electrolysis. We will look at three. Ionic compounds conduct electricity when molten or in solution. Ionic compounds conduct electricity when molten or in solution. Think of electrolysis and electrolytic cells as. Why We Use Electrodes In Electrolysis.
From spmscience.blog.onlinetuition.com.my
Electrolysis SPM Science Why We Use Electrodes In Electrolysis Ionic compounds conduct electricity when molten or in solution. In an electrolytic cell, however, the. Reactive metals are extracted from their ores using electrolysis. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. We will look at three. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Electrolysis, process. Why We Use Electrodes In Electrolysis.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Why We Use Electrodes In Electrolysis In an electrolytic cell, however, the. A chemical change takes place when atoms and ions lose or gain electrons. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. We will look at three. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a. Why We Use Electrodes In Electrolysis.
From general.chemistrysteps.com
Electrolysis Chemistry Steps Why We Use Electrodes In Electrolysis Reactive metals are extracted from their ores using electrolysis. In an electrolytic cell, however, the. A chemical change takes place when atoms and ions lose or gain electrons. Ionic compounds conduct electricity when molten or in solution. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. We will look at three. Reactive. Why We Use Electrodes In Electrolysis.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Why We Use Electrodes In Electrolysis Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. A chemical change takes place when atoms and ions. Why We Use Electrodes In Electrolysis.
From saylordotorg.github.io
Electrolysis Why We Use Electrodes In Electrolysis Reactive metals are extracted from their ores using electrolysis. A chemical change takes place when atoms and ions lose or gain electrons. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Ionic compounds conduct electricity when. Why We Use Electrodes In Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 Why We Use Electrodes In Electrolysis Reactive metals are extracted from their ores using electrolysis. Reactive metals are extracted from their ores using electrolysis. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Think of electrolysis and. Why We Use Electrodes In Electrolysis.