What Is Chemistry Equilibrium . chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. Chemical equilibrium is a dynamic process,. — a reaction is at equilibrium when the amounts of reactants or products no longer change. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a.
from general.chemistrysteps.com
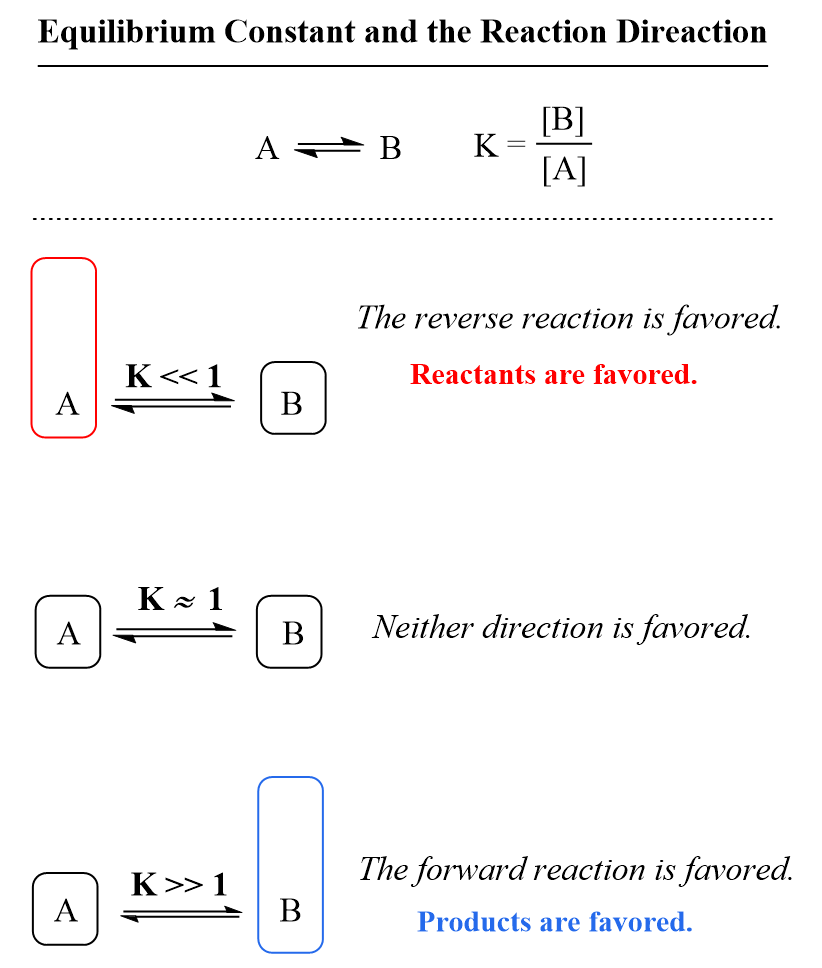
chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. — a reaction is at equilibrium when the amounts of reactants or products no longer change. Chemical equilibrium is a dynamic process,.
Equilibrium Constant Chemistry Steps
What Is Chemistry Equilibrium — a reaction is at equilibrium when the amounts of reactants or products no longer change. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. Chemical equilibrium is a dynamic process,. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. — a reaction is at equilibrium when the amounts of reactants or products no longer change. reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a.
From www.pinterest.com
Chemical Equilibrium Types, Problems, Factors Affecting, Examples What Is Chemistry Equilibrium — a reaction is at equilibrium when the amounts of reactants or products no longer change. reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and.. What Is Chemistry Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Is Chemistry Equilibrium reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. Chemical equilibrium is a dynamic process,. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. — a reaction is at equilibrium when the amounts of. What Is Chemistry Equilibrium.
From study.com
Dynamic & Chemical Equilibrium Definition & Examples Lesson What Is Chemistry Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. — a reaction is at equilibrium when the amounts of reactants or products no longer change. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and. What Is Chemistry Equilibrium.
From www.slideserve.com
PPT Chapter 7 Chemical Equilibrium PowerPoint Presentation, free What Is Chemistry Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. — a reaction is at equilibrium when the amounts of reactants or products. What Is Chemistry Equilibrium.
From gamesmartz.com
Law Of Chemical Equilibrium Definition Easy to Understand What Is Chemistry Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of. What Is Chemistry Equilibrium.
From www.youtube.com
Understanding The Equilibrium Constant, Kp (A2 Chemistry) YouTube What Is Chemistry Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. Chemical equilibrium is a dynamic process,. chemical equilibrium is a dynamic process that. What Is Chemistry Equilibrium.
From thechemistrynotes.com
Chemical Equilibrium Definition, Types, Importance, and Examples What Is Chemistry Equilibrium Chemical equilibrium is a dynamic process,. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. chemical equilibrium is a dynamic process that consists of a. What Is Chemistry Equilibrium.
From mungfali.com
Types Of Equilibrium Chemistry What Is Chemistry Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. Chemical equilibrium is a dynamic process,. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. chemical equilibrium is a dynamic process that. What Is Chemistry Equilibrium.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID What Is Chemistry Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. — a reaction is at equilibrium when the amounts of reactants or products no longer. What Is Chemistry Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Is Chemistry Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no. What Is Chemistry Equilibrium.
From www.youtube.com
What is Chemical Equilibrium Explain with Example Class 10 Chemistry What Is Chemistry Equilibrium — a reaction is at equilibrium when the amounts of reactants or products no longer change. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. Chemical equilibrium. What Is Chemistry Equilibrium.
From www.youtube.com
The equilibrium law. A chemistry tutorial. YouTube What Is Chemistry Equilibrium reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in. What Is Chemistry Equilibrium.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID What Is Chemistry Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. Chemical equilibrium is a dynamic process,. reactions reach chemical equilibrium when the rate of the. What Is Chemistry Equilibrium.
From artofsmart.com.au
Guide to HSC Chemistry Module 5 Equilibrium and Acid Reactions What Is Chemistry Equilibrium Chemical equilibrium is a dynamic process,. — a reaction is at equilibrium when the amounts of reactants or products no longer change. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. chemical equilibrium is a dynamic process that consists of a forward reaction, in which. What Is Chemistry Equilibrium.
From studylib.net
Chemical Equilibrium Introduction What Is Chemistry Equilibrium — a reaction is at equilibrium when the amounts of reactants or products no longer change. Chemical equilibrium is a dynamic process,. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. reactions reach chemical equilibrium when the rate of the forward reaction equals. What Is Chemistry Equilibrium.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent What Is Chemistry Equilibrium — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. reactions reach chemical equilibrium when the rate of the forward reaction equals the. What Is Chemistry Equilibrium.
From www.pinterest.com
Chemical Equilibrium at a Glance Teaching chemistry, Chemistry What Is Chemistry Equilibrium reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. Chemical equilibrium is a dynamic process,. — a reaction is at equilibrium when the amounts of reactants or products no longer change. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted. What Is Chemistry Equilibrium.
From www.chemistrylearner.com
Chemical Equilibrium Definition, Principles, and Examples What Is Chemistry Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. reactions reach chemical equilibrium when the rate of the forward reaction equals the. What Is Chemistry Equilibrium.
From www.vedantu.com
Equilibrium Class 11 Notes for NEET Chemistry Revision What Is Chemistry Equilibrium reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. Chemical equilibrium is a dynamic process,. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. — a reaction is at equilibrium when the amounts of reactants or. What Is Chemistry Equilibrium.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and What Is Chemistry Equilibrium — a reaction is at equilibrium when the amounts of reactants or products no longer change. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and. What Is Chemistry Equilibrium.
From www.expii.com
What Is Equilibrium? — Definition & Overview Expii What Is Chemistry Equilibrium reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to. What Is Chemistry Equilibrium.
From www.shalom-education.com
Dynamic Equilibrium GCSE Chemistry Revision What Is Chemistry Equilibrium Chemical equilibrium is a dynamic process,. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. — a reaction is at equilibrium when the amounts of reactants or products no longer change. chemical equilibrium is a dynamic process that consists of a forward reaction,. What Is Chemistry Equilibrium.
From www.chemicals.co.uk
Chemistry A Level Revision Equilibrium What Is Chemistry Equilibrium — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. Chemical equilibrium is a dynamic process,. — a reaction is at equilibrium when the amounts of reactants or products no longer change. chemical equilibrium is a dynamic process that consists of a forward reaction,. What Is Chemistry Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Is Chemistry Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. Chemical equilibrium is a dynamic process,. reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. chemical equilibrium is a dynamic process that consists of a forward reaction,. What Is Chemistry Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Is Chemistry Equilibrium — a reaction is at equilibrium when the amounts of reactants or products no longer change. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. Chemical equilibrium. What Is Chemistry Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Is Chemistry Equilibrium reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. Chemical equilibrium is a dynamic process,. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. — a reaction is at equilibrium when the amounts of reactants or. What Is Chemistry Equilibrium.
From learningchemistryeasily.blogspot.com
Learning Chemistry Easily Chemical Equilibrium Theory, An Introduction What Is Chemistry Equilibrium — a reaction is at equilibrium when the amounts of reactants or products no longer change. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. Chemical equilibrium is a dynamic process,. — chemical equilibrium, condition in the course of a reversible chemical reaction in which. What Is Chemistry Equilibrium.
From general.chemistrysteps.com
Equilibrium Constant Chemistry Steps What Is Chemistry Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in. What Is Chemistry Equilibrium.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM Chapter 16 PowerPoint Presentation, free What Is Chemistry Equilibrium reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. — a reaction is at equilibrium when the amounts of reactants or products no longer change. —. What Is Chemistry Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Is Chemistry Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in. What Is Chemistry Equilibrium.
From chemistrynotes.com
What is Chemical Equilibrium? What Is Chemistry Equilibrium Chemical equilibrium is a dynamic process,. — a reaction is at equilibrium when the amounts of reactants or products no longer change. reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted. What Is Chemistry Equilibrium.
From www.bartleby.com
Chemical Equilibrium bartleby What Is Chemistry Equilibrium — a reaction is at equilibrium when the amounts of reactants or products no longer change. Chemical equilibrium is a dynamic process,. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. chemical equilibrium is a dynamic process that consists of a forward reaction,. What Is Chemistry Equilibrium.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM Unit 11, Part II PowerPoint Presentation What Is Chemistry Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. — a reaction is at equilibrium when the amounts of reactants or products no longer. What Is Chemistry Equilibrium.
From www.len.com.ng
Chemical Equilibrium Dynamic Equilibrium in Chemistry What Is Chemistry Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a. reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of. What Is Chemistry Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Is Chemistry Equilibrium Chemical equilibrium is a dynamic process,. — chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. chemical equilibrium is a dynamic process that consists of a. What Is Chemistry Equilibrium.