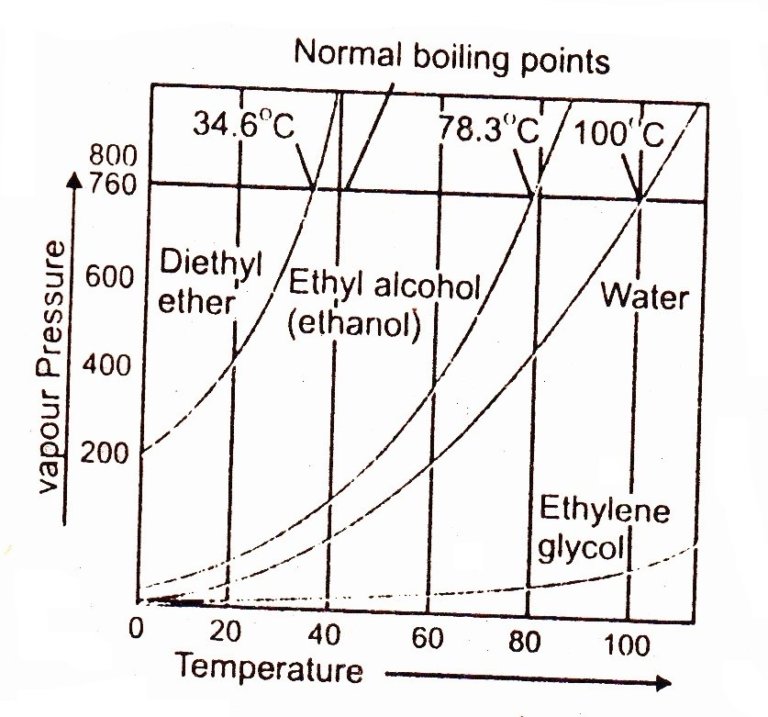
Describe Boiling Point . The normal boiling point of water is 100°c at 1 atm, but it can vary with altitude. Learn how boiling point depends on pressure and temperature, and how to use it for purification and cooking. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. At sea level, water boils at 100° c (212° f), but at higher. Find out the factors that affect the boiling point of a substance and see examples of different boiling. Learn what boiling point is and how it relates to the vapor pressure of liquids. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Learn the definition, examples, and factors that affect the boiling point of liquids. Normal boiling point is the temperature at which. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. The boiling point depends on. Learn how boiling points are affected by pressure, purity,.
from chemistryskills.com
At sea level, water boils at 100° c (212° f), but at higher. Learn the definition, examples, and factors that affect the boiling point of liquids. Normal boiling point is the temperature at which. Learn what boiling point is and how it relates to the vapor pressure of liquids. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The normal boiling point of water is 100°c at 1 atm, but it can vary with altitude. The boiling point depends on. Learn how boiling point depends on pressure and temperature, and how to use it for purification and cooking. Learn how boiling points are affected by pressure, purity,.
Definition and Explanation of Boiling Point Chemistry Skills
Describe Boiling Point Learn what boiling point is and how it relates to the vapor pressure of liquids. The normal boiling point of water is 100°c at 1 atm, but it can vary with altitude. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Normal boiling point is the temperature at which. At sea level, water boils at 100° c (212° f), but at higher. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. Learn the definition, examples, and factors that affect the boiling point of liquids. Learn how boiling point depends on pressure and temperature, and how to use it for purification and cooking. Learn what boiling point is and how it relates to the vapor pressure of liquids. Find out the factors that affect the boiling point of a substance and see examples of different boiling. The boiling point depends on. Learn how boiling points are affected by pressure, purity,.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin Describe Boiling Point At sea level, water boils at 100° c (212° f), but at higher. Learn how boiling points are affected by pressure, purity,. Find out the factors that affect the boiling point of a substance and see examples of different boiling. The boiling point depends on. Learn the definition, examples, and factors that affect the boiling point of liquids. Learn what. Describe Boiling Point.
From gamesmartz.com
Boiling Point Definition & Image GameSmartz Describe Boiling Point Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. Normal boiling point is the temperature at which. Learn the definition, examples, and factors that affect the boiling point of liquids. At sea level, water boils at 100° c (212° f), but at higher. Find out the factors that affect the boiling point. Describe Boiling Point.
From www.youtube.com
22 Boiling Point Elevation Explanation YouTube Describe Boiling Point Normal boiling point is the temperature at which. Learn how boiling point depends on pressure and temperature, and how to use it for purification and cooking. Learn the definition, examples, and factors that affect the boiling point of liquids. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Boiling point. Describe Boiling Point.
From socratic.org
What is the boiling point (in °C) of a 1.56 m aqueous solution of CaCl Describe Boiling Point The normal boiling point of water is 100°c at 1 atm, but it can vary with altitude. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Find out the factors that affect the boiling point of a substance. Describe Boiling Point.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation ID1115834 Describe Boiling Point Normal boiling point is the temperature at which. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn how boiling point depends on pressure and temperature, and how to use it for purification and cooking. Learn how boiling points are affected by pressure, purity,. Learn the definition, examples, and. Describe Boiling Point.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry Describe Boiling Point Learn how boiling points are affected by pressure, purity,. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. The normal boiling point of water is 100°c at 1 atm, but it can. Describe Boiling Point.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills Describe Boiling Point Find out the factors that affect the boiling point of a substance and see examples of different boiling. Learn how boiling point depends on pressure and temperature, and how to use it for purification and cooking. Normal boiling point is the temperature at which. Boiling is the process by which a liquid turns into a vapor when it is heated. Describe Boiling Point.
From www.differencebetween.com
Difference Between Boiling Point and Melting Point Compare the Describe Boiling Point Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn how boiling points are affected by pressure, purity,. The normal boiling point of water is 100°c at 1 atm, but it can vary with altitude. Learn what boiling point is and how it relates to the vapor pressure of. Describe Boiling Point.
From www.animalia-life.club
Boiling Point Of Water Examples Describe Boiling Point Learn the definition, examples, and factors that affect the boiling point of liquids. Learn what boiling point is and how it relates to the vapor pressure of liquids. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The boiling point depends on. Normal boiling point is the temperature at which.. Describe Boiling Point.
From www.youtube.com
What is the Difference Between Boiling Point and Condensing Point Describe Boiling Point Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn how boiling points are affected by pressure, purity,. Learn what boiling point is and how it relates to the vapor pressure of liquids. The normal boiling point of water is 100°c at 1 atm, but it can vary with. Describe Boiling Point.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID1624745 Describe Boiling Point At sea level, water boils at 100° c (212° f), but at higher. Learn how boiling point depends on pressure and temperature, and how to use it for purification and cooking. The normal boiling point of water is 100°c at 1 atm, but it can vary with altitude. Boiling point is the temperature where a liquid's vapor pressure equals its. Describe Boiling Point.
From www.thoughtco.com
Normal Boiling Point Definition (Chemistry) Describe Boiling Point Find out the factors that affect the boiling point of a substance and see examples of different boiling. Normal boiling point is the temperature at which. The boiling point depends on. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Boiling is the process by which a liquid turns into. Describe Boiling Point.
From www.thermoworks.com
Thermal Secrets to Boiling Point Calibration ThermoWorks Describe Boiling Point The boiling point depends on. Find out the factors that affect the boiling point of a substance and see examples of different boiling. Learn how boiling point depends on pressure and temperature, and how to use it for purification and cooking. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling. Describe Boiling Point.
From imgbin.com
Boiling Point Melting Point Heat Temperature Chemistry PNG, Clipart Describe Boiling Point The boiling point depends on. Learn how boiling point depends on pressure and temperature, and how to use it for purification and cooking. Learn the definition, examples, and factors that affect the boiling point of liquids. Learn what boiling point is and how it relates to the vapor pressure of liquids. At sea level, water boils at 100° c (212°. Describe Boiling Point.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID Describe Boiling Point At sea level, water boils at 100° c (212° f), but at higher. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Boiling is the process by which a liquid turns into a. Describe Boiling Point.
From klaubgmaz.blob.core.windows.net
What Is Boiling Point In Science at Lan Johnson blog Describe Boiling Point At sea level, water boils at 100° c (212° f), but at higher. The boiling point depends on. Learn how boiling point depends on pressure and temperature, and how to use it for purification and cooking. Learn the definition, examples, and factors that affect the boiling point of liquids. Boiling point is the temperature where a liquid's vapor pressure equals. Describe Boiling Point.
From www.thoughtco.com
Definition of Boiling Point in Chemistry Describe Boiling Point Find out the factors that affect the boiling point of a substance and see examples of different boiling. The normal boiling point of water is 100°c at 1 atm, but it can vary with altitude. The boiling point depends on. Learn what boiling point is and how it relates to the vapor pressure of liquids. At sea level, water boils. Describe Boiling Point.
From www.chegg.com
Solved Define boiling point. Why can the boiling point of a Describe Boiling Point Learn what boiling point is and how it relates to the vapor pressure of liquids. Learn the definition, examples, and factors that affect the boiling point of liquids. The boiling point depends on. At sea level, water boils at 100° c (212° f), but at higher. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure.. Describe Boiling Point.
From ar.inspiredpencil.com
Boiling Point Of Water Examples Describe Boiling Point Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn what boiling point is and how it relates to the vapor pressure of liquids. Learn how boiling points are affected by pressure, purity,. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Normal boiling. Describe Boiling Point.
From klaubgmaz.blob.core.windows.net
What Is Boiling Point In Science at Lan Johnson blog Describe Boiling Point Learn what boiling point is and how it relates to the vapor pressure of liquids. Learn the definition, examples, and factors that affect the boiling point of liquids. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. At sea level, water boils at 100° c (212° f), but at. Describe Boiling Point.
From exomkbouq.blob.core.windows.net
What Is Boiling Point Elevation Constant at Ronnie Moore blog Describe Boiling Point Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. The normal boiling point of water is 100°c at 1 atm, but it can vary with altitude. Learn how boiling point depends on pressure and temperature, and how to use it for purification and cooking. Normal boiling point is the temperature at which.. Describe Boiling Point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Describe Boiling Point Learn how boiling points are affected by pressure, purity,. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn what boiling point is and how it relates to the vapor pressure of liquids. At sea level, water boils at 100° c (212° f), but at higher. Boiling point is. Describe Boiling Point.
From brainly.in
Define melting point, boiling point, latent heat of fusion, latent heat Describe Boiling Point At sea level, water boils at 100° c (212° f), but at higher. Learn what boiling point is and how it relates to the vapor pressure of liquids. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its. Describe Boiling Point.
From en.ppt-online.org
The alkali metals online presentation Describe Boiling Point Find out the factors that affect the boiling point of a substance and see examples of different boiling. Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Normal boiling point is the temperature at which. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. Learn how boiling. Describe Boiling Point.
From byjus.com
Determination Of Boiling Point Of An Organic Compound Chemistry Describe Boiling Point Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. At sea level, water boils at 100° c (212° f), but at higher. Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. Learn what boiling point is and how it relates to. Describe Boiling Point.
From labonlaptop.com
Find Boiling Point of Water labOnLaptop Store Describe Boiling Point The normal boiling point of water is 100°c at 1 atm, but it can vary with altitude. Find out the factors that affect the boiling point of a substance and see examples of different boiling. The boiling point depends on. Learn the definition, examples, and factors that affect the boiling point of liquids. Boiling point is the temperature where a. Describe Boiling Point.
From chemistnotes.com
Boiling Point Definition, Boiling point as physical properties Describe Boiling Point Learn how boiling point depends on pressure and temperature, and how to use it for purification and cooking. Find out the factors that affect the boiling point of a substance and see examples of different boiling. The boiling point depends on. Learn the definition, examples, and factors that affect the boiling point of liquids. Boiling is the process by which. Describe Boiling Point.
From www.youtube.com
Melting Point, Boiling Point and Freezing Point Chemistry YouTube Describe Boiling Point Learn how boiling points are affected by pressure, purity,. At sea level, water boils at 100° c (212° f), but at higher. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Find out the factors that affect the boiling point of a substance and see examples of different boiling.. Describe Boiling Point.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry Describe Boiling Point Learn what boiling point is and how it relates to the vapor pressure of liquids. At sea level, water boils at 100° c (212° f), but at higher. Find out the factors that affect the boiling point of a substance and see examples of different boiling. Boiling point is the temperature at which the vapor pressure of a liquid equals. Describe Boiling Point.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID6901289 Describe Boiling Point Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Find out the factors that affect the boiling point of a substance and see examples of different boiling. At sea level, water boils at 100° c (212°. Describe Boiling Point.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Describe Boiling Point Boiling point is the temperature where a liquid's vapor pressure equals its applied pressure. Find out the factors that affect the boiling point of a substance and see examples of different boiling. Learn how boiling point depends on pressure and temperature, and how to use it for purification and cooking. Learn how boiling points are affected by pressure, purity,. Learn. Describe Boiling Point.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy Describe Boiling Point Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. The normal boiling point of water is 100°c at 1 atm, but it can vary with altitude. Learn what boiling point is and how it relates to the vapor pressure of liquids. At sea level, water boils at 100° c (212° f), but. Describe Boiling Point.
From www.chegg.com
Solved Define boiling point and describe how it can change Describe Boiling Point Learn how boiling point depends on pressure and temperature, and how to use it for purification and cooking. The normal boiling point of water is 100°c at 1 atm, but it can vary with altitude. Normal boiling point is the temperature at which. Learn how boiling points are affected by pressure, purity,. Boiling is the process by which a liquid. Describe Boiling Point.
From loeaanczy.blob.core.windows.net
Chlorine Boiling Point And Melting Point at Jane Gibbs blog Describe Boiling Point Boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Find out the factors that affect the boiling point of a substance and see examples of different boiling. Boiling point is the temperature. Describe Boiling Point.
From www.showme.com
ShowMe boiling point elevation Describe Boiling Point Find out the factors that affect the boiling point of a substance and see examples of different boiling. The boiling point depends on. Learn what boiling point is and how it relates to the vapor pressure of liquids. Learn how boiling points are affected by pressure, purity,. The normal boiling point of water is 100°c at 1 atm, but it. Describe Boiling Point.