Device Labeling Requirements . Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Manufacturer’s name and business location. General labeling requirements are defined in cfr title 21, part 801. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Regulations on unique device identification management, etc.pdf.
from www.greenlight.guru
Manufacturer’s name and business location. General labeling requirements are defined in cfr title 21, part 801. Regulations on unique device identification management, etc.pdf. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to.
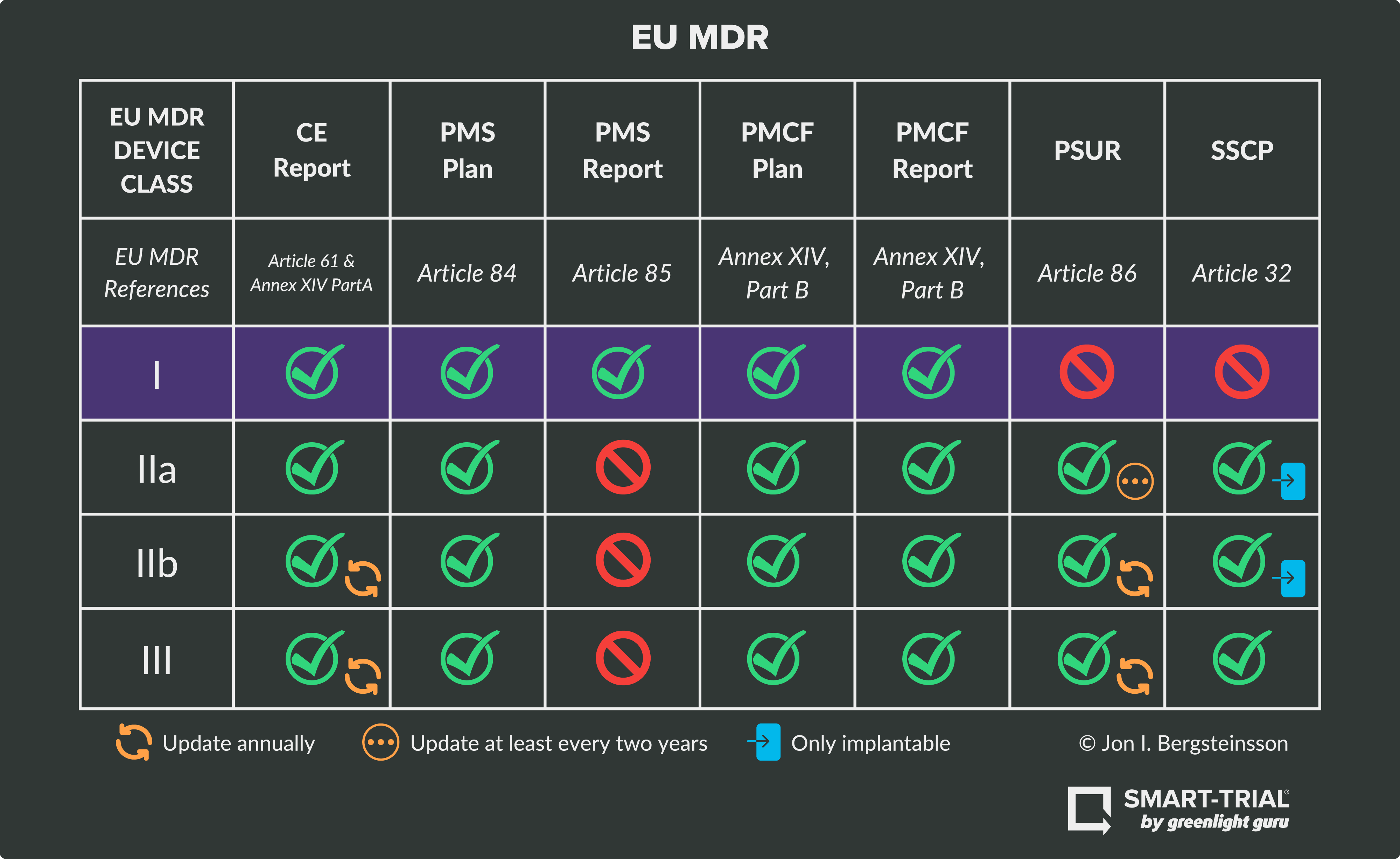
Ultimate Guide to Device Class Requirements under EU MDR
Device Labeling Requirements Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Manufacturer’s name and business location. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Regulations on unique device identification management, etc.pdf. General labeling requirements are defined in cfr title 21, part 801.
From www.freseniusmedicalcare.com
Medical device regulation Fresenius Medical Care Device Labeling Requirements Regulations on unique device identification management, etc.pdf. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Manufacturer’s name and business location. General labeling requirements are defined in cfr. Device Labeling Requirements.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Device Labeling Requirements Manufacturer’s name and business location. General labeling requirements are defined in cfr title 21, part 801. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Regulations on unique. Device Labeling Requirements.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Device Labeling Requirements General labeling requirements are defined in cfr title 21, part 801. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Manufacturer’s name and business location. Regulations on unique device identification management, etc.pdf. Elements of medical device and ivd medical device labelling ras require and specify information that. Device Labeling Requirements.
From klaiqkgel.blob.core.windows.net
Medical Device Labeling Requirements Australia at Peter Rameriz blog Device Labeling Requirements General labeling requirements are defined in cfr title 21, part 801. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Regulations on unique device identification management, etc.pdf. Manufacturer’s name and business location. Elements of medical device and ivd medical device labelling ras require and specify information that. Device Labeling Requirements.
From www.linkedin.com
Complying with Medical Device Labeling Requirements Device Labeling Requirements Regulations on unique device identification management, etc.pdf. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). General labeling requirements are defined in cfr title 21, part 801. Manufacturer’s name and business location. Elements of medical device and ivd medical device labelling ras require and specify information that. Device Labeling Requirements.
From klaiqkgel.blob.core.windows.net
Medical Device Labeling Requirements Australia at Peter Rameriz blog Device Labeling Requirements Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). General labeling requirements are defined in cfr title 21, part 801. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Manufacturer’s name and business location. Regulations on unique. Device Labeling Requirements.
From hiveta.com
Label Compliance AB&R® (American Barcode and RFID) Device Labeling Requirements Regulations on unique device identification management, etc.pdf. General labeling requirements are defined in cfr title 21, part 801. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Manufacturer’s. Device Labeling Requirements.
From mungfali.com
FDA Medical Device Label Symbols Device Labeling Requirements General labeling requirements are defined in cfr title 21, part 801. Regulations on unique device identification management, etc.pdf. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Manufacturer’s name and business location. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code. Device Labeling Requirements.
From www.scribd.com
General Device Labeling Requirements PDF PDF Government Business Device Labeling Requirements General labeling requirements are defined in cfr title 21, part 801. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Regulations on unique device identification management, etc.pdf. Manufacturer’s name and business location. Elements of medical device and ivd medical device labelling ras require and specify information that. Device Labeling Requirements.
From klaiqkgel.blob.core.windows.net
Medical Device Labeling Requirements Australia at Peter Rameriz blog Device Labeling Requirements Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Regulations on unique device identification management, etc.pdf. Manufacturer’s name and business location. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). General labeling requirements are defined in cfr. Device Labeling Requirements.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Device Labeling Requirements Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Manufacturer’s name and business location. Regulations on unique device identification management, etc.pdf. General labeling requirements are defined in cfr title 21, part 801. Elements of medical device and ivd medical device labelling ras require and specify information that. Device Labeling Requirements.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Device Labeling Requirements General labeling requirements are defined in cfr title 21, part 801. Manufacturer’s name and business location. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Regulations on unique device identification management, etc.pdf. Elements of medical device and ivd medical device labelling ras require and specify information that. Device Labeling Requirements.
From andamanmed.com
Medical device labeling requirements in the Philippines Device Labeling Requirements Regulations on unique device identification management, etc.pdf. General labeling requirements are defined in cfr title 21, part 801. Manufacturer’s name and business location. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Elements of medical device and ivd medical device labelling ras require and specify information that. Device Labeling Requirements.
From www.scribd.com
FDA Medical Device Labeling Requirements Checklist Greenlight Guru PDF Device Labeling Requirements Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Manufacturer’s name and business location. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). General labeling requirements are defined in cfr title 21, part 801. Regulations on unique. Device Labeling Requirements.
From www.afpharmaservice.com
Medical Device Labelling Requirements Device Labeling Requirements Manufacturer’s name and business location. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. General labeling requirements are defined in cfr title 21, part 801. Regulations on unique device identification management, etc.pdf. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code. Device Labeling Requirements.
From issuu.com
Medical Device Labeling Requirements VISTAAR by VISTAAR Issuu Device Labeling Requirements General labeling requirements are defined in cfr title 21, part 801. Manufacturer’s name and business location. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Regulations on unique. Device Labeling Requirements.
From www.schlafenderhase.com
Medical Device Labeling Requirements Schlafender Hase Device Labeling Requirements Regulations on unique device identification management, etc.pdf. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Manufacturer’s name and business location. General labeling requirements are defined in cfr. Device Labeling Requirements.
From medenvoyglobal.com
Medical Device Labeling Requirements in Europe MedEnvoy Device Labeling Requirements Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Regulations on unique device identification management, etc.pdf. General labeling requirements are defined in cfr title 21, part 801. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Manufacturer’s. Device Labeling Requirements.
From klaiqkgel.blob.core.windows.net
Medical Device Labeling Requirements Australia at Peter Rameriz blog Device Labeling Requirements Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Regulations on unique device identification management, etc.pdf. Manufacturer’s name and business location. General labeling requirements are defined in cfr title 21, part 801. Elements of medical device and ivd medical device labelling ras require and specify information that. Device Labeling Requirements.
From satoasiapacific.com
SATO Medical Device Barcode Labelling Solution SATO AutoID Malaysia Device Labeling Requirements Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Regulations on unique device identification management, etc.pdf. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Manufacturer’s name and business location. General labeling requirements are defined in cfr. Device Labeling Requirements.
From www.greenlight.guru
FDA Medical Device Labeling Requirements An Overview Device Labeling Requirements Manufacturer’s name and business location. Regulations on unique device identification management, etc.pdf. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. General labeling requirements are defined in cfr title 21, part 801. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code. Device Labeling Requirements.
From cexdbwxv.blob.core.windows.net
Fda Label Font Requirements at Toby Martinez blog Device Labeling Requirements Regulations on unique device identification management, etc.pdf. Manufacturer’s name and business location. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. General labeling requirements are defined in cfr title 21, part 801. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code. Device Labeling Requirements.
From ar.inspiredpencil.com
Fda Labeling Regulations Device Labeling Requirements Regulations on unique device identification management, etc.pdf. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Manufacturer’s name and business location. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. General labeling requirements are defined in cfr. Device Labeling Requirements.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Device Labeling Requirements Regulations on unique device identification management, etc.pdf. Manufacturer’s name and business location. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. General labeling requirements are defined in cfr. Device Labeling Requirements.
From klaiqkgel.blob.core.windows.net
Medical Device Labeling Requirements Australia at Peter Rameriz blog Device Labeling Requirements General labeling requirements are defined in cfr title 21, part 801. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Regulations on unique device identification management, etc.pdf. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Manufacturer’s. Device Labeling Requirements.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Device Labeling Requirements Manufacturer’s name and business location. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Regulations on unique device identification management, etc.pdf. General labeling requirements are defined in cfr title 21, part 801. Elements of medical device and ivd medical device labelling ras require and specify information that. Device Labeling Requirements.
From joiyedckh.blob.core.windows.net
Chemical Labeling Requirements at Heather Keller blog Device Labeling Requirements Manufacturer’s name and business location. Regulations on unique device identification management, etc.pdf. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). General labeling requirements are defined in cfr. Device Labeling Requirements.
From exyzsultp.blob.core.windows.net
Fda Guidance Medical Device Patient Labeling at Jana Flores blog Device Labeling Requirements General labeling requirements are defined in cfr title 21, part 801. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Regulations on unique device identification management, etc.pdf. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Manufacturer’s. Device Labeling Requirements.
From ceoavkzr.blob.core.windows.net
Ukraine Medical Device Labeling Requirements at Myron Hallenbeck blog Device Labeling Requirements Manufacturer’s name and business location. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Regulations on unique device identification management, etc.pdf. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. General labeling requirements are defined in cfr. Device Labeling Requirements.
From www.mastertrial.com
MDR Requirements for Device Labeling and Implant Card Mastertrial Device Labeling Requirements General labeling requirements are defined in cfr title 21, part 801. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Regulations on unique device identification management, etc.pdf. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Manufacturer’s. Device Labeling Requirements.
From www.greenlight.guru
Ultimate Guide to Device Class Requirements under EU MDR Device Labeling Requirements Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Manufacturer’s name and business location. General labeling requirements are defined in cfr title 21, part 801. Regulations on unique device identification management, etc.pdf. Elements of medical device and ivd medical device labelling ras require and specify information that. Device Labeling Requirements.
From www.greenlight.guru
Medical Device Labeling Definition & Requirements Device Labeling Requirements Manufacturer’s name and business location. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). General labeling requirements are defined in cfr title 21, part 801. Regulations on unique device identification management, etc.pdf. Elements of medical device and ivd medical device labelling ras require and specify information that. Device Labeling Requirements.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Device Labeling Requirements Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). General labeling requirements are defined in cfr title 21, part 801. Regulations on unique device identification management, etc.pdf. Manufacturer’s name and business location. Elements of medical device and ivd medical device labelling ras require and specify information that. Device Labeling Requirements.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Device Labeling Requirements Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Manufacturer’s name and business location. Regulations on unique device identification management, etc.pdf. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). General labeling requirements are defined in cfr. Device Labeling Requirements.
From www.youtube.com
FDA Requirements for Device Labeling YouTube Device Labeling Requirements Regulations on unique device identification management, etc.pdf. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). General labeling requirements are defined in cfr title 21, part 801. Manufacturer’s name and business location. Elements of medical device and ivd medical device labelling ras require and specify information that. Device Labeling Requirements.