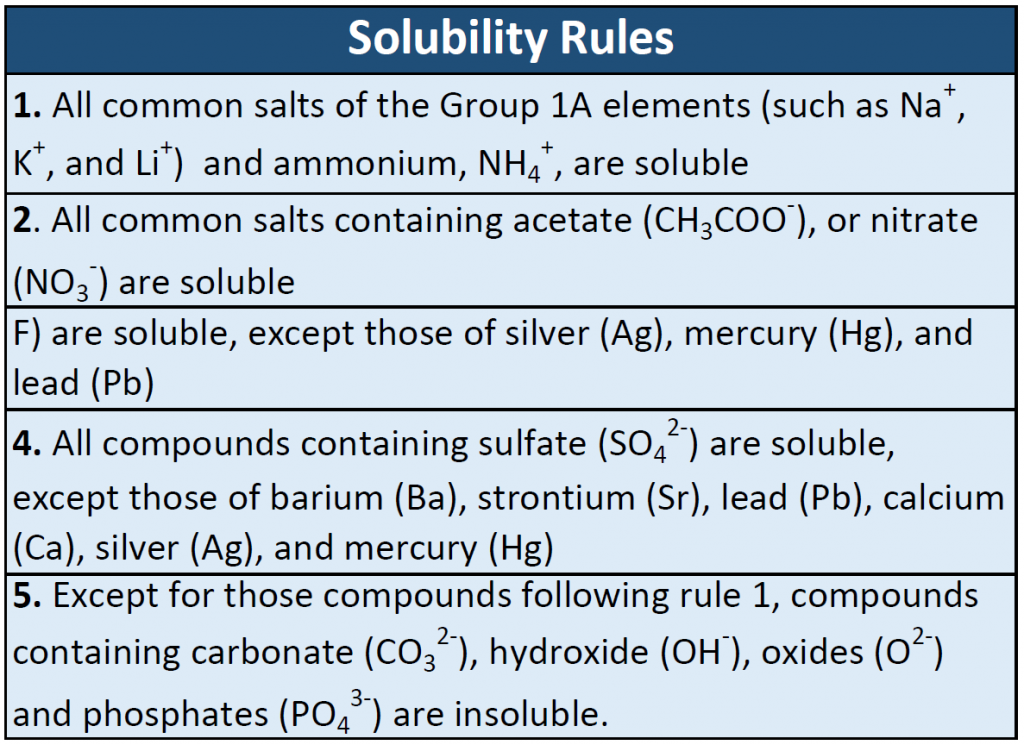
What Compound Is Most Soluble In Water And Why . predict solubilities of ionic compounds in water using the solubility rules. All nitrates are soluble in water. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. The extent to which a substance may be dissolved in. Sr 3 (po 4) 2; these are the general solubility rules for inorganic compounds, primarily inorganic salts. classify each compound as soluble or insoluble. Aliphatic and aromatic compounds are. Use the solubility rules to determine whether a. It has more number of bonds of hydrogen, hence, greater. the types of compounds that are soluble in water include ionic compounds and polar compounds.
from wou.edu
Aliphatic and aromatic compounds are. Sr 3 (po 4) 2; the types of compounds that are soluble in water include ionic compounds and polar compounds. ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. The extent to which a substance may be dissolved in. these are the general solubility rules for inorganic compounds, primarily inorganic salts. It has more number of bonds of hydrogen, hence, greater. classify each compound as soluble or insoluble. predict solubilities of ionic compounds in water using the solubility rules. All nitrates are soluble in water.
CH104 Chapter 7 Solutions Chemistry
What Compound Is Most Soluble In Water And Why Aliphatic and aromatic compounds are. ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. classify each compound as soluble or insoluble. The extent to which a substance may be dissolved in. the types of compounds that are soluble in water include ionic compounds and polar compounds. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. It has more number of bonds of hydrogen, hence, greater. Aliphatic and aromatic compounds are. predict solubilities of ionic compounds in water using the solubility rules. these are the general solubility rules for inorganic compounds, primarily inorganic salts. All nitrates are soluble in water. Use the solubility rules to determine whether a. Sr 3 (po 4) 2;
From exogqaefj.blob.core.windows.net
Copper Chloride Water Solubility at Sandra Barrera blog What Compound Is Most Soluble In Water And Why It has more number of bonds of hydrogen, hence, greater. Aliphatic and aromatic compounds are. predict solubilities of ionic compounds in water using the solubility rules. the types of compounds that are soluble in water include ionic compounds and polar compounds. All nitrates are soluble in water. ethylene glycol compound consists of two hydroxy groups that form. What Compound Is Most Soluble In Water And Why.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID2493612 What Compound Is Most Soluble In Water And Why a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. The extent to which a substance may be dissolved in. It has more number of bonds of hydrogen, hence, greater. ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds. What Compound Is Most Soluble In Water And Why.
From www.chegg.com
Solved O Arrange the compounds from most soluble in water to What Compound Is Most Soluble In Water And Why The extent to which a substance may be dissolved in. Sr 3 (po 4) 2; predict solubilities of ionic compounds in water using the solubility rules. It has more number of bonds of hydrogen, hence, greater. classify each compound as soluble or insoluble. ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water.. What Compound Is Most Soluble In Water And Why.
From www.chegg.com
Solved Rank These Compounds From Most Soluble In Water To... What Compound Is Most Soluble In Water And Why the types of compounds that are soluble in water include ionic compounds and polar compounds. All nitrates are soluble in water. these are the general solubility rules for inorganic compounds, primarily inorganic salts. Sr 3 (po 4) 2; a salt is soluble if it dissolves in water to give a solution with a concentration of at least. What Compound Is Most Soluble In Water And Why.
From ar.inspiredpencil.com
Solubility In Water What Compound Is Most Soluble In Water And Why these are the general solubility rules for inorganic compounds, primarily inorganic salts. It has more number of bonds of hydrogen, hence, greater. Sr 3 (po 4) 2; classify each compound as soluble or insoluble. The extent to which a substance may be dissolved in. ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with. What Compound Is Most Soluble In Water And Why.
From casegrocooley.blogspot.com
Determine Whether Each Compound Is Soluble or Insoluble in Water What Compound Is Most Soluble In Water And Why It has more number of bonds of hydrogen, hence, greater. classify each compound as soluble or insoluble. these are the general solubility rules for inorganic compounds, primarily inorganic salts. Sr 3 (po 4) 2; The extent to which a substance may be dissolved in. Use the solubility rules to determine whether a. the types of compounds that. What Compound Is Most Soluble In Water And Why.
From www.chegg.com
Solved Which compound will be the most soluble in water? What Compound Is Most Soluble In Water And Why classify each compound as soluble or insoluble. ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. All nitrates are soluble in water. predict solubilities of ionic compounds in water using the solubility rules. these are the general solubility rules for inorganic compounds, primarily inorganic salts. a salt is soluble if. What Compound Is Most Soluble In Water And Why.
From www.slideserve.com
PPT Solubility of organic compound organic chemistry II lab What Compound Is Most Soluble In Water And Why the types of compounds that are soluble in water include ionic compounds and polar compounds. predict solubilities of ionic compounds in water using the solubility rules. The extent to which a substance may be dissolved in. classify each compound as soluble or insoluble. Aliphatic and aromatic compounds are. It has more number of bonds of hydrogen, hence,. What Compound Is Most Soluble In Water And Why.
From www.numerade.com
SOLVED Arrange the following compounds according to their solubility What Compound Is Most Soluble In Water And Why Use the solubility rules to determine whether a. Sr 3 (po 4) 2; ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. All nitrates are soluble in water. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room. What Compound Is Most Soluble In Water And Why.
From www.chegg.com
Solved Which of the following compounds will be most soluble What Compound Is Most Soluble In Water And Why predict solubilities of ionic compounds in water using the solubility rules. Aliphatic and aromatic compounds are. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. classify each compound as soluble or insoluble. The extent to which a substance may be. What Compound Is Most Soluble In Water And Why.
From www.youtube.com
Why are ionic compounds soluble in water? YouTube What Compound Is Most Soluble In Water And Why predict solubilities of ionic compounds in water using the solubility rules. All nitrates are soluble in water. ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. Use the solubility rules to determine whether a. the types of compounds that are soluble in water include ionic compounds and polar compounds. a salt. What Compound Is Most Soluble In Water And Why.
From ulisesfersburns.blogspot.com
Identify Which of the Following Is Soluble in Water. What Compound Is Most Soluble In Water And Why Aliphatic and aromatic compounds are. these are the general solubility rules for inorganic compounds, primarily inorganic salts. The extent to which a substance may be dissolved in. Sr 3 (po 4) 2; It has more number of bonds of hydrogen, hence, greater. ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. All nitrates. What Compound Is Most Soluble In Water And Why.
From www.chegg.com
Solved Which compound is the most soluble in water? O What Compound Is Most Soluble In Water And Why the types of compounds that are soluble in water include ionic compounds and polar compounds. It has more number of bonds of hydrogen, hence, greater. The extent to which a substance may be dissolved in. classify each compound as soluble or insoluble. All nitrates are soluble in water. Use the solubility rules to determine whether a. predict. What Compound Is Most Soluble In Water And Why.
From webmis.highland.cc.il.us
Aqueous Solutions What Compound Is Most Soluble In Water And Why All nitrates are soluble in water. classify each compound as soluble or insoluble. It has more number of bonds of hydrogen, hence, greater. these are the general solubility rules for inorganic compounds, primarily inorganic salts. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per. What Compound Is Most Soluble In Water And Why.
From www.youtube.com
which molecule is most soluble in water? YouTube What Compound Is Most Soluble In Water And Why these are the general solubility rules for inorganic compounds, primarily inorganic salts. All nitrates are soluble in water. It has more number of bonds of hydrogen, hence, greater. ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. Aliphatic and aromatic compounds are. Sr 3 (po 4) 2; The extent to which a substance. What Compound Is Most Soluble In Water And Why.
From www.chegg.com
Solved Which compound is the most soluble in water? What Compound Is Most Soluble In Water And Why It has more number of bonds of hydrogen, hence, greater. The extent to which a substance may be dissolved in. the types of compounds that are soluble in water include ionic compounds and polar compounds. classify each compound as soluble or insoluble. Use the solubility rules to determine whether a. ethylene glycol compound consists of two hydroxy. What Compound Is Most Soluble In Water And Why.
From www.bartleby.com
Answered 1. Which molecule is most soluble in… bartleby What Compound Is Most Soluble In Water And Why All nitrates are soluble in water. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. predict solubilities of ionic compounds in water using the solubility rules. The extent to which a substance may be dissolved in. It has more number of. What Compound Is Most Soluble In Water And Why.
From www.numerade.com
SOLVED Which compound is the MOST soluble in water? OH "OH What Compound Is Most Soluble In Water And Why ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. predict solubilities of ionic compounds in water using the solubility rules. these are the general solubility rules for inorganic compounds, primarily inorganic salts. The extent to which a substance may be dissolved in. All nitrates are soluble in water. Sr 3 (po 4). What Compound Is Most Soluble In Water And Why.
From solvedlib.com
Which of the following compounds is most soluble in w… SolvedLib What Compound Is Most Soluble In Water And Why ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. Sr 3 (po 4) 2; predict solubilities of ionic compounds in water using the solubility rules. It has more number of bonds of hydrogen, hence, greater. Aliphatic and aromatic compounds are. Use the solubility rules to determine whether a. the types of compounds. What Compound Is Most Soluble In Water And Why.
From www.youtube.com
Predicting solubility of ionic compounds in water YouTube What Compound Is Most Soluble In Water And Why Use the solubility rules to determine whether a. these are the general solubility rules for inorganic compounds, primarily inorganic salts. All nitrates are soluble in water. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. predict solubilities of ionic compounds. What Compound Is Most Soluble In Water And Why.
From www.chegg.com
Solved Which Compound Will Be Most Soluble In Water? Whic... What Compound Is Most Soluble In Water And Why Use the solubility rules to determine whether a. classify each compound as soluble or insoluble. It has more number of bonds of hydrogen, hence, greater. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. Aliphatic and aromatic compounds are. the. What Compound Is Most Soluble In Water And Why.
From www.slideserve.com
PPT Reaction Stoichiometry Mole Method Calculations PowerPoint What Compound Is Most Soluble In Water And Why predict solubilities of ionic compounds in water using the solubility rules. ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. the types of compounds that are soluble in water include ionic compounds and polar compounds. All nitrates are soluble in water. these are the general solubility rules for inorganic compounds, primarily. What Compound Is Most Soluble In Water And Why.
From wou.edu
CH104 Chapter 7 Solutions Chemistry What Compound Is Most Soluble In Water And Why The extent to which a substance may be dissolved in. classify each compound as soluble or insoluble. All nitrates are soluble in water. Use the solubility rules to determine whether a. It has more number of bonds of hydrogen, hence, greater. ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. these are. What Compound Is Most Soluble In Water And Why.
From www.breakingatom.com
Solubility of Elements and Compounds What Compound Is Most Soluble In Water And Why classify each compound as soluble or insoluble. Aliphatic and aromatic compounds are. All nitrates are soluble in water. these are the general solubility rules for inorganic compounds, primarily inorganic salts. the types of compounds that are soluble in water include ionic compounds and polar compounds. ethylene glycol compound consists of two hydroxy groups that form hydrogen. What Compound Is Most Soluble In Water And Why.
From www.chegg.com
Solved Which compound is the most soluble in water? O What Compound Is Most Soluble In Water And Why a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. The extent to which a substance may be dissolved in. It has more number of bonds of hydrogen, hence, greater. classify each compound as soluble or insoluble. Sr 3 (po 4) 2;. What Compound Is Most Soluble In Water And Why.
From www.numerade.com
SOLVEDIdentify the compound in each group that is most soluble in What Compound Is Most Soluble In Water And Why predict solubilities of ionic compounds in water using the solubility rules. ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. Use the solubility rules to determine whether a. All nitrates are soluble in water. Aliphatic and aromatic compounds are. the types of compounds that are soluble in water include ionic compounds and. What Compound Is Most Soluble In Water And Why.
From www.chegg.com
Solved Arrange the compounds from most soluble in water to What Compound Is Most Soluble In Water And Why Use the solubility rules to determine whether a. predict solubilities of ionic compounds in water using the solubility rules. Aliphatic and aromatic compounds are. the types of compounds that are soluble in water include ionic compounds and polar compounds. these are the general solubility rules for inorganic compounds, primarily inorganic salts. Sr 3 (po 4) 2; The. What Compound Is Most Soluble In Water And Why.
From wou.edu
CH104 Chapter 7 Solutions Chemistry What Compound Is Most Soluble In Water And Why The extent to which a substance may be dissolved in. It has more number of bonds of hydrogen, hence, greater. Use the solubility rules to determine whether a. the types of compounds that are soluble in water include ionic compounds and polar compounds. Sr 3 (po 4) 2; ethylene glycol compound consists of two hydroxy groups that form. What Compound Is Most Soluble In Water And Why.
From www.coursehero.com
[Solved] how to determine most soluble in water? Course Hero What Compound Is Most Soluble In Water And Why The extent to which a substance may be dissolved in. All nitrates are soluble in water. classify each compound as soluble or insoluble. Sr 3 (po 4) 2; ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. It has more number of bonds of hydrogen, hence, greater. a salt is soluble if. What Compound Is Most Soluble In Water And Why.
From www.chemistrysteps.com
Solubility of Organic Compounds Chemistry Steps What Compound Is Most Soluble In Water And Why predict solubilities of ionic compounds in water using the solubility rules. Sr 3 (po 4) 2; ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. Aliphatic and aromatic compounds are. It has more number of bonds of hydrogen, hence, greater. All nitrates are soluble in water. the types of compounds that are. What Compound Is Most Soluble In Water And Why.
From casegrocooley.blogspot.com
Determine Whether Each Compound Is Soluble or Insoluble in Water What Compound Is Most Soluble In Water And Why predict solubilities of ionic compounds in water using the solubility rules. these are the general solubility rules for inorganic compounds, primarily inorganic salts. Aliphatic and aromatic compounds are. Sr 3 (po 4) 2; The extent to which a substance may be dissolved in. a salt is soluble if it dissolves in water to give a solution with. What Compound Is Most Soluble In Water And Why.
From www.numerade.com
SOLVED Arrange the organic compounds from most soluble in water t0 What Compound Is Most Soluble In Water And Why a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. Sr 3 (po 4) 2; Aliphatic and aromatic compounds are. these are the general solubility rules for inorganic compounds, primarily inorganic salts. ethylene glycol compound consists of two hydroxy groups that. What Compound Is Most Soluble In Water And Why.
From www.chegg.com
Solved Arrange the compounds from most soluble in water to What Compound Is Most Soluble In Water And Why Use the solubility rules to determine whether a. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. Aliphatic and aromatic compounds are. these are the general solubility rules for inorganic compounds, primarily inorganic salts. the types of compounds that are. What Compound Is Most Soluble In Water And Why.
From study.com
Compound Solubility in Water Overview & Examples Lesson What Compound Is Most Soluble In Water And Why predict solubilities of ionic compounds in water using the solubility rules. The extent to which a substance may be dissolved in. It has more number of bonds of hydrogen, hence, greater. Sr 3 (po 4) 2; Aliphatic and aromatic compounds are. ethylene glycol compound consists of two hydroxy groups that form hydrogen bonds with water. a salt. What Compound Is Most Soluble In Water And Why.
From www.chegg.com
Solved Choose the compound that is the most soluble in What Compound Is Most Soluble In Water And Why these are the general solubility rules for inorganic compounds, primarily inorganic salts. The extent to which a substance may be dissolved in. All nitrates are soluble in water. predict solubilities of ionic compounds in water using the solubility rules. classify each compound as soluble or insoluble. It has more number of bonds of hydrogen, hence, greater. Sr. What Compound Is Most Soluble In Water And Why.