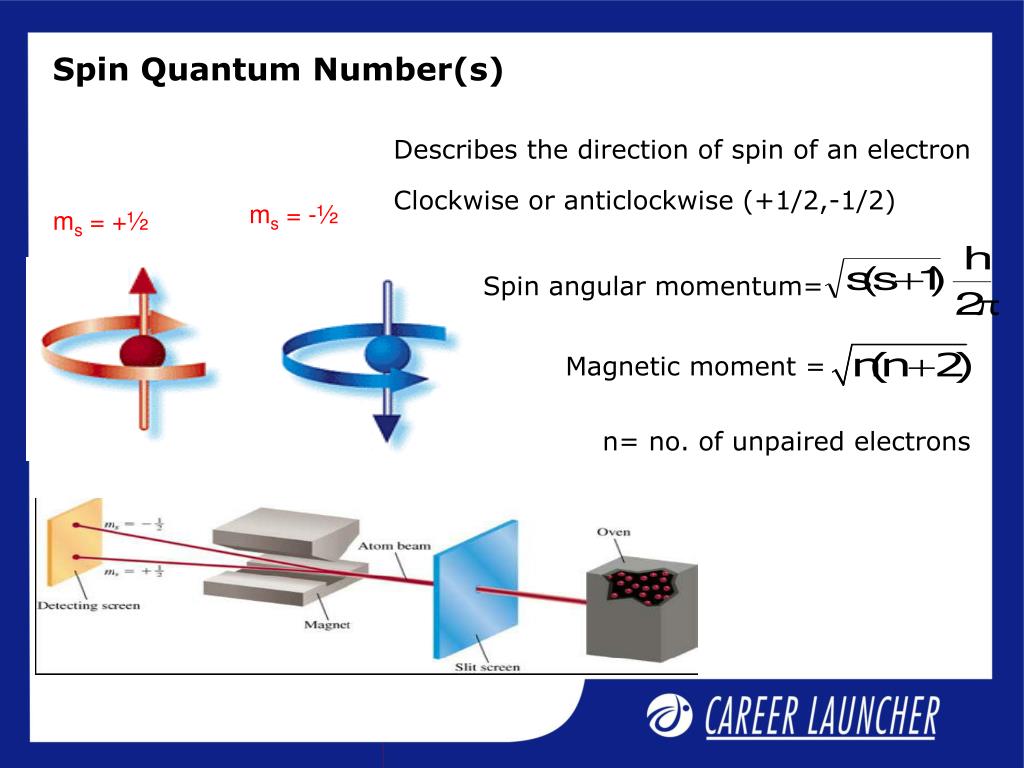
Difference Between Magnetic Quantum Number And Spin Quantum Number . The possible ml values follow the. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. An electron spins around an. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the value ml is the magnetic quantum number. the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. the principal quantum number is one of three quantum numbers used to characterize an orbital. An atomic orbital, which is distinct from an orbit, is a. the magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (zeeman effect), while the spin quantum number.
from www.slideserve.com
the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. The possible ml values follow the. the principal quantum number is one of three quantum numbers used to characterize an orbital. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (zeeman effect), while the spin quantum number. An atomic orbital, which is distinct from an orbit, is a. An electron spins around an. the value ml is the magnetic quantum number.
PPT Chemistry PowerPoint Presentation, free download ID834035
Difference Between Magnetic Quantum Number And Spin Quantum Number the principal quantum number is one of three quantum numbers used to characterize an orbital. the magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (zeeman effect), while the spin quantum number. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. the principal quantum number is one of three quantum numbers used to characterize an orbital. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. An electron spins around an. the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. The possible ml values follow the. An atomic orbital, which is distinct from an orbit, is a. the value ml is the magnetic quantum number.
From www.chemistrylearner.com
Spin Quantum Number Definition, Significance, and Value Difference Between Magnetic Quantum Number And Spin Quantum Number An electron spins around an. the principal quantum number is one of three quantum numbers used to characterize an orbital. the value ml is the magnetic quantum number. The possible ml values follow the. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. the spin quantum number. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From chemistnotes.com
Quantum Numbers, Types Principal, Azimuthal, and Spin Difference Between Magnetic Quantum Number And Spin Quantum Number An atomic orbital, which is distinct from an orbit, is a. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (zeeman. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Unit 2 PowerPoint Presentation, free download ID298336 Difference Between Magnetic Quantum Number And Spin Quantum Number the principal quantum number is one of three quantum numbers used to characterize an orbital. the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the magnetic quantum number helps explain the splitting of spectral. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin Difference Between Magnetic Quantum Number And Spin Quantum Number the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (zeeman effect), while the spin quantum number. the value ml. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.priyamstudycentre.com
Quantum Number Orbitals Diagram, Definition, Chart, Shape Difference Between Magnetic Quantum Number And Spin Quantum Number the value ml is the magnetic quantum number. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. An electron spins around an. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. An atomic orbital, which is distinct from an orbit, is a. the magnetic. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID2135857 Difference Between Magnetic Quantum Number And Spin Quantum Number The possible ml values follow the. the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. the principal quantum number is one of three quantum numbers used to characterize an orbital. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the magnetic quantum number,. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Quantum Theory and Electron Configurations PowerPoint Difference Between Magnetic Quantum Number And Spin Quantum Number An atomic orbital, which is distinct from an orbit, is a. The possible ml values follow the. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. the spin quantum. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From isemprole.my.to
Quantum Numbers Principal, Azimuthal, & Spin Difference Between Magnetic Quantum Number And Spin Quantum Number The possible ml values follow the. An electron spins around an. the value ml is the magnetic quantum number. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. . Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.differencebetween.com
Difference Between Quantum Number and Spin Quantum Number Difference Between Magnetic Quantum Number And Spin Quantum Number the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. the magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (zeeman effect), while the spin quantum number. the principal quantum number is one of three quantum numbers used to characterize an orbital. . Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Unit 2 PowerPoint Presentation, free download ID298336 Difference Between Magnetic Quantum Number And Spin Quantum Number the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. the principal quantum number is one of. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin Difference Between Magnetic Quantum Number And Spin Quantum Number the magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (zeeman effect), while the spin quantum number. An electron spins around an. An atomic orbital, which is distinct from an orbit, is a. The possible ml values follow the. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID4952601 Difference Between Magnetic Quantum Number And Spin Quantum Number An electron spins around an. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. An atomic orbital, which is distinct from an orbit, is a. the value ml is the magnetic quantum number. the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field,. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Chapter 28 PowerPoint Presentation, free download ID1104026 Difference Between Magnetic Quantum Number And Spin Quantum Number The possible ml values follow the. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. An atomic orbital, which is distinct from an orbit, is a. the principal quantum number is one of three quantum numbers used to characterize an orbital. An electron spins around an. the magnetic quantum number determines the orientation of. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Principle Quantum Numbers PowerPoint Presentation, free download Difference Between Magnetic Quantum Number And Spin Quantum Number the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. An atomic orbital, which is distinct from an orbit, is a. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the magnetic quantum number, symbolized as m l, is an integral value that determines the. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From derifit.blogspot.com
What Does Spin Quantum Number Determine DERIFIT Difference Between Magnetic Quantum Number And Spin Quantum Number the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. The possible ml values follow the. the principal quantum number is one of three quantum numbers used to characterize an orbital. the magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (zeeman effect), while. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From protonstalk.com
Four Quantum Numbers Significance and FAQs ProtonsTalk Difference Between Magnetic Quantum Number And Spin Quantum Number the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. An electron spins around an. The possible ml values follow the. the value ml is the magnetic quantum number. the magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (zeeman effect), while the. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From relationshipbetween.com
Difference Between Quantum Number And Spin Quantum Number Difference Between Magnetic Quantum Number And Spin Quantum Number the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. the magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (zeeman effect), while the spin quantum number. the value ml is the magnetic quantum number. the spin quantum number (\(m_s\)) describes the. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From slideplayer.com
Quantum Numbers. ppt download Difference Between Magnetic Quantum Number And Spin Quantum Number the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. the value ml is the magnetic quantum number. the principal quantum number is one of three quantum numbers used to characterize an orbital. The possible ml values follow the. the spin quantum number (\(m_s\)) describes the angular momentum. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Chapter Seven Atomic Structure PowerPoint Presentation, free Difference Between Magnetic Quantum Number And Spin Quantum Number the principal quantum number is one of three quantum numbers used to characterize an orbital. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. the magnetic quantum number helps explain the splitting of spectral lines. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From relationshipbetween.com
Difference Between Quantum Number And Spin Quantum Number Difference Between Magnetic Quantum Number And Spin Quantum Number the principal quantum number is one of three quantum numbers used to characterize an orbital. An atomic orbital, which is distinct from an orbit, is a. An electron spins around an. the magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (zeeman effect), while the spin quantum number. the magnetic quantum number,. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Spin Quantum Number, m s PowerPoint Presentation, free download Difference Between Magnetic Quantum Number And Spin Quantum Number the magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (zeeman effect), while the spin quantum number. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. The possible ml values follow the. An electron spins around an. the value ml is the magnetic. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Quantum Numbers Activity PowerPoint Presentation, free download Difference Between Magnetic Quantum Number And Spin Quantum Number the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. The possible ml values follow the. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. An atomic orbital, which is distinct from an orbit, is a. the magnetic quantum number determines the orientation of an electron's. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Atomic Quantum Numbers PowerPoint Presentation, free download Difference Between Magnetic Quantum Number And Spin Quantum Number the spin quantum number (\(m_s\)) describes the angular momentum of an electron. The possible ml values follow the. the principal quantum number is one of three quantum numbers used to characterize an orbital. the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. the magnetic quantum number,. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From chemistnotes.com
Quantum Numbers, Types Principal, Azimuthal, and Spin Difference Between Magnetic Quantum Number And Spin Quantum Number the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the value ml is the magnetic quantum number. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. the principal quantum number is one of three quantum numbers used to characterize an orbital. the magnetic. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.differencebetween.com
Difference Between Quantum Number and Spin Quantum Number Difference Between Magnetic Quantum Number And Spin Quantum Number the principal quantum number is one of three quantum numbers used to characterize an orbital. the value ml is the magnetic quantum number. the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. the magnetic quantum number, symbolized as m l, is an integral value that determines. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Chemistry 101 Chap. 6 PowerPoint Presentation, free download Difference Between Magnetic Quantum Number And Spin Quantum Number An atomic orbital, which is distinct from an orbit, is a. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. The possible ml values follow the. the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. An electron spins around an. the magnetic quantum number,. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Atomic Structure and Periodicity PowerPoint Presentation, free Difference Between Magnetic Quantum Number And Spin Quantum Number the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. The possible ml values follow the. An atomic orbital, which is distinct from an orbit, is a. the magnetic quantum number determines the orientation of an electron's. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.youtube.com
Quantum Number,Spin Quantum number. YouTube Difference Between Magnetic Quantum Number And Spin Quantum Number An atomic orbital, which is distinct from an orbit, is a. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. the magnetic quantum number helps explain the splitting of. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation ID2135857 Difference Between Magnetic Quantum Number And Spin Quantum Number An electron spins around an. An atomic orbital, which is distinct from an orbit, is a. the value ml is the magnetic quantum number. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field,. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID834035 Difference Between Magnetic Quantum Number And Spin Quantum Number the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. the principal quantum number is one of three quantum numbers used to characterize an orbital. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the magnetic quantum number helps explain the splitting of spectral. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Arrangement of Electrons in Atoms PowerPoint Presentation, free Difference Between Magnetic Quantum Number And Spin Quantum Number the principal quantum number is one of three quantum numbers used to characterize an orbital. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the value ml is the magnetic quantum number. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. The possible ml. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.youtube.com
Quantum Numbers of Electrons (Principle, Azimuthal, Spin Difference Between Magnetic Quantum Number And Spin Quantum Number the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the magnetic quantum number, symbolized as m l, is an integral value that determines the orientation of an. An electron spins around an. An atomic orbital, which is distinct from an orbit, is a. the magnetic quantum number determines the orientation of an electron's orbital. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID9555840 Difference Between Magnetic Quantum Number And Spin Quantum Number The possible ml values follow the. the magnetic quantum number determines the orientation of an electron's orbital in a magnetic field, while the spin quantum. the magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (zeeman effect), while the spin quantum number. the principal quantum number is one of three quantum numbers. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From education-portal.com
Spin Quantum Number Definition & Example Video & Lesson Transcript Difference Between Magnetic Quantum Number And Spin Quantum Number An electron spins around an. the value ml is the magnetic quantum number. the principal quantum number is one of three quantum numbers used to characterize an orbital. the magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (zeeman effect), while the spin quantum number. the spin quantum number (\(m_s\)) describes. Difference Between Magnetic Quantum Number And Spin Quantum Number.
From www.slideserve.com
PPT Chapter 6 Electronic Structure of Atoms PowerPoint Presentation Difference Between Magnetic Quantum Number And Spin Quantum Number the magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (zeeman effect), while the spin quantum number. the value ml is the magnetic quantum number. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. the magnetic quantum number determines the orientation of an electron's orbital in a magnetic. Difference Between Magnetic Quantum Number And Spin Quantum Number.