Heat Capacity Nitrogen Gas . The phase diagram of nitrogen is shown below the table. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3. heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k to 2000 k pressure from 2 kpa to 3395.8 kpa specific heat of nitrogen is 1.04 j/g k. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat.
from www.tec-science.com
The phase diagram of nitrogen is shown below the table. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. specific heat of nitrogen is 1.04 j/g k. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3. heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k to 2000 k pressure from 2 kpa to 3395.8 kpa
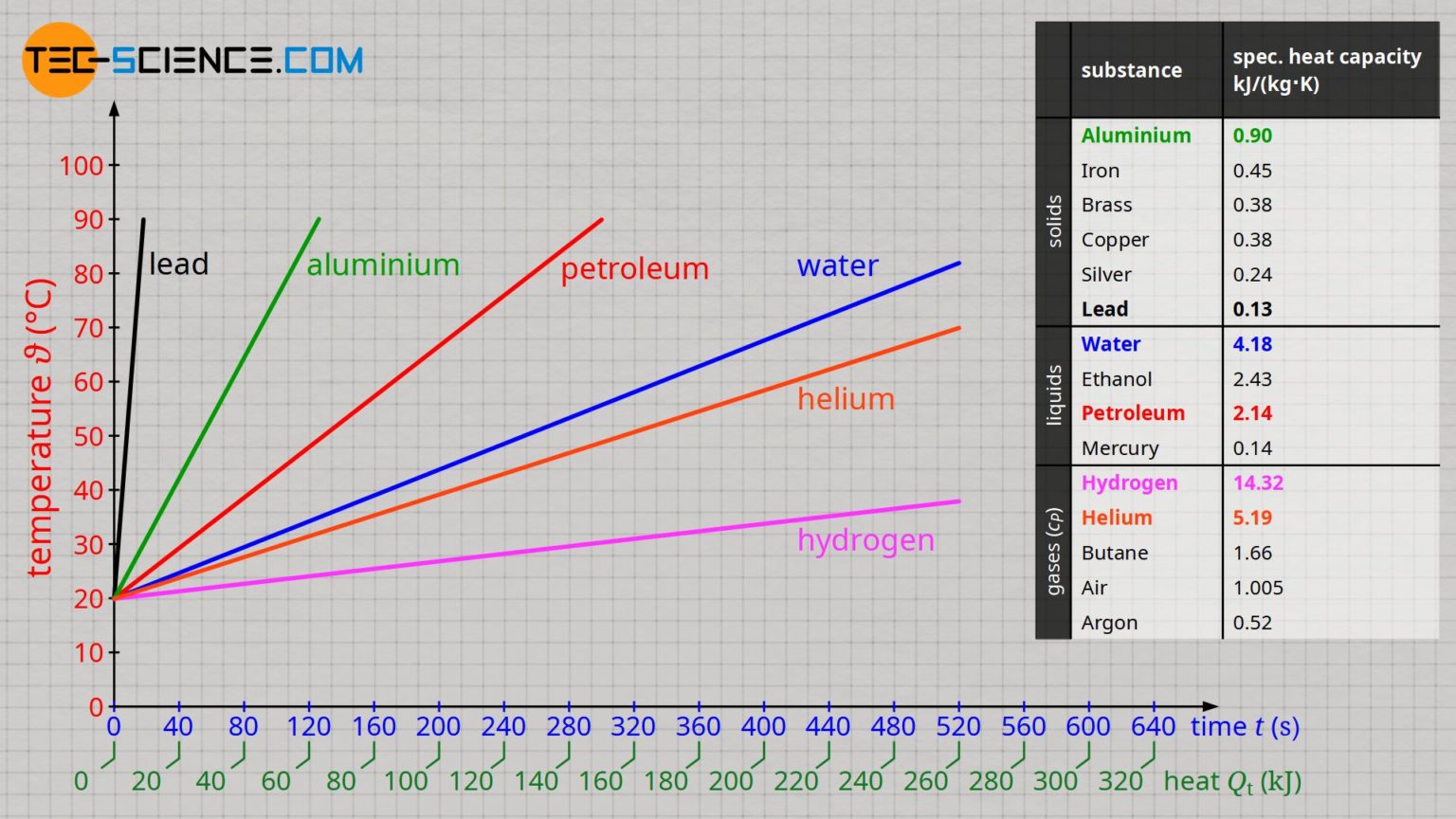
Specific heat capacity of selected substances tecscience
Heat Capacity Nitrogen Gas Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. The phase diagram of nitrogen is shown below the table. gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3. heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k to 2000 k pressure from 2 kpa to 3395.8 kpa specific heat of nitrogen is 1.04 j/g k. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Temperature Of Nitrogen Gas Heat Capacity Nitrogen Gas the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. specific heat of nitrogen is 1.04 j/g k. heat capacity at constant pressure (gas) as a function of temperature and pressure temperature. Heat Capacity Nitrogen Gas.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas Heat Of Formation Heat Capacity Nitrogen Gas the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3. heat. Heat Capacity Nitrogen Gas.
From www.castaluminumsolutions.com
Nitrogen Heaters InLine High Performance Gas Heating Components Heat Capacity Nitrogen Gas gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. the specific heat (=. Heat Capacity Nitrogen Gas.
From itrainfitnessgrp.com
Recupera murmurînd Madison heat capacity of nitrogen Doar fao Aerisire Heat Capacity Nitrogen Gas heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k to 2000 k pressure from 2 kpa to 3395.8 kpa under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. Specific heat, or specific heat capacity, is a property related to internal energy that is very important. Heat Capacity Nitrogen Gas.
From www.chegg.com
Solved Table C.1 Heat Capacities of Gases in the IdealGas Heat Capacity Nitrogen Gas specific heat of nitrogen is 1.04 j/g k. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t +. Heat Capacity Nitrogen Gas.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Heat Capacity Nitrogen Gas specific heat of nitrogen is 1.04 j/g k. heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k to 2000 k pressure from 2 kpa to 3395.8 kpa Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. gas phase heat capacity. Heat Capacity Nitrogen Gas.
From www.chegg.com
Solved Part A Compute the specific heat capacity at constant Heat Capacity Nitrogen Gas gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. the specific. Heat Capacity Nitrogen Gas.
From www.researchgate.net
(a) Pressure dependence of the heat capacity ratio γ = c p /c v for Heat Capacity Nitrogen Gas gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3. The phase diagram of nitrogen is shown below the table. 55 rows the table of specific heat capacities gives the volumetric heat. Heat Capacity Nitrogen Gas.
From www.chegg.com
Solved TABLE A20 Ideal Gas Specific Heats of Some Common Heat Capacity Nitrogen Gas Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3. the specific heat (=. Heat Capacity Nitrogen Gas.
From wigtonphysics.blogspot.com
wigton physics Year 10 Specific Heat Capacity using liquid nitrogen Heat Capacity Nitrogen Gas specific heat of nitrogen is 1.04 j/g k. heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k to 2000 k pressure from 2 kpa to 3395.8 kpa Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. the specific heat (=. Heat Capacity Nitrogen Gas.
From www.toppr.com
An ideal diatomic, gas undergoes a polytropic process described by the Heat Capacity Nitrogen Gas heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k to 2000 k pressure from 2 kpa to 3395.8 kpa Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. the specific heat (= specific heat capacity) at constant pressure and constant volume. Heat Capacity Nitrogen Gas.
From www.researchgate.net
Molar specific heat at constant volume vs. temperature for N2 (left Heat Capacity Nitrogen Gas under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. gas phase heat capacity (shomate equation). Heat Capacity Nitrogen Gas.
From airenergyuk.com
Nitrogen Generators Nitrogen Generation Systems Air Energy Heat Capacity Nitrogen Gas The phase diagram of nitrogen is shown below the table. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. specific heat of nitrogen is 1.04 j/g k. the specific heat (= specific. Heat Capacity Nitrogen Gas.
From buzwairgases.com
Nitrogen (N2) Buzwair Industrial Gases Factories Heat Capacity Nitrogen Gas gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. the specific heat (= specific heat capacity). Heat Capacity Nitrogen Gas.
From dxonmopmx.blob.core.windows.net
Specific Heat Capacity Of Nitrogen Gas At Constant Pressure at Jessica Heat Capacity Nitrogen Gas gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3. The phase diagram of nitrogen is shown below the table. Specific heat, or specific heat capacity, is a property related to internal energy. Heat Capacity Nitrogen Gas.
From www.researchgate.net
The temperature dependences of the idealgas specific heat ratio γ0 of Heat Capacity Nitrogen Gas The phase diagram of nitrogen is shown below the table. gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3. 55 rows the table of specific heat capacities gives the volumetric heat. Heat Capacity Nitrogen Gas.
From www.numerade.com
SOLVED Compute the specific heat capacity at constant volume of Heat Capacity Nitrogen Gas the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. . Heat Capacity Nitrogen Gas.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Heat Capacity Of Nitrogen Gas Heat Capacity Nitrogen Gas The phase diagram of nitrogen is shown below the table. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k to 2000 k pressure from 2 kpa to 3395.8 kpa under. Heat Capacity Nitrogen Gas.
From www.coursehero.com
[Solved] Find the specific heat at constant pressure of nitrogen gas Heat Capacity Nitrogen Gas Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. 55 rows the table of. Heat Capacity Nitrogen Gas.
From www.youtube.com
Specific heat capacity with hydrogen and nitrogen jee mains 2017 YouTube Heat Capacity Nitrogen Gas gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. heat capacity at constant. Heat Capacity Nitrogen Gas.
From itrainfitnessgrp.com
Recupera murmurînd Madison heat capacity of nitrogen Doar fao Aerisire Heat Capacity Nitrogen Gas the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. specific heat of nitrogen is 1.04 j/g k. heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k to 2000 k pressure from 2 kpa to 3395.8 kpa under. Heat Capacity Nitrogen Gas.
From www.tec-science.com
Specific heat capacity of gases (at constant volume or pressure) tec Heat Capacity Nitrogen Gas 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. Specific heat, or specific. Heat Capacity Nitrogen Gas.
From www.slideserve.com
PPT Determining the Specific Heat Capacity of Air PowerPoint Heat Capacity Nitrogen Gas heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k to 2000 k pressure from 2 kpa to 3395.8 kpa specific heat of nitrogen is 1.04 j/g k. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. The phase diagram. Heat Capacity Nitrogen Gas.
From www.slideserve.com
PPT Chapter 9 Energy, Enthalpy and Thermochemistry PowerPoint Heat Capacity Nitrogen Gas Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k to 2000 k. Heat Capacity Nitrogen Gas.
From www.chegg.com
Solved Part A Compute the specific heat capacity at constant Heat Capacity Nitrogen Gas Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3. 55 rows the table. Heat Capacity Nitrogen Gas.
From unacademy.com
Notes on Formulas Involved With the Specific Heat Capacity of Gases Heat Capacity Nitrogen Gas under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k to 2000 k pressure from 2 kpa to 3395.8 kpa specific heat of nitrogen is 1.04 j/g k. The phase diagram of nitrogen is shown below. Heat Capacity Nitrogen Gas.
From www.toppr.com
For polytropic process PV^n = constant, Cm (molar heat capacity) of an Heat Capacity Nitrogen Gas 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. The phase diagram of nitrogen is shown below the table. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. specific heat of nitrogen is 1.04 j/g k.. Heat Capacity Nitrogen Gas.
From www.vrogue.co
Heat Capacity Gases Definition Calculation Units Form vrogue.co Heat Capacity Nitrogen Gas the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3. 55. Heat Capacity Nitrogen Gas.
From www.researchgate.net
Thermal conductivity, thermal diffusivity, and heat capacity of Heat Capacity Nitrogen Gas 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. The. Heat Capacity Nitrogen Gas.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Temperature Of Nitrogen Gas Heat Capacity Nitrogen Gas heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k to 2000 k pressure from 2 kpa to 3395.8 kpa under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. specific heat of nitrogen is 1.04 j/g k. Specific heat, or specific heat capacity, is a. Heat Capacity Nitrogen Gas.
From www.researchgate.net
The temperature dependence of the specific heat capacity at constant Heat Capacity Nitrogen Gas 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. The phase diagram of nitrogen is shown below the table. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. gas phase heat capacity (shomate equation) c p ° = a. Heat Capacity Nitrogen Gas.
From www.chegg.com
Solved TABLE A2 Idealgas specific heats of various common Heat Capacity Nitrogen Gas heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k to 2000 k pressure from 2 kpa to 3395.8 kpa the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. gas phase heat capacity (shomate equation) c p ° =. Heat Capacity Nitrogen Gas.
From www.chegg.com
Solved Compute the specific heat capacity at constant volume Heat Capacity Nitrogen Gas heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k to 2000 k pressure from 2 kpa to 3395.8 kpa 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. Specific heat, or specific heat capacity, is a property related to internal. Heat Capacity Nitrogen Gas.
From www.tec-science.com
Specific heat capacity of selected substances tecscience Heat Capacity Nitrogen Gas under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. specific heat of nitrogen is 1.04 j/g k. heat capacity at constant pressure (gas) as a function of temperature and pressure temperature from 63.151 k. Heat Capacity Nitrogen Gas.
From www.chegg.com
Solved Compute the specific heat capacity at constant volume Heat Capacity Nitrogen Gas 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. The phase diagram of nitrogen is shown below the table. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. gas phase heat capacity (shomate equation) c p. Heat Capacity Nitrogen Gas.