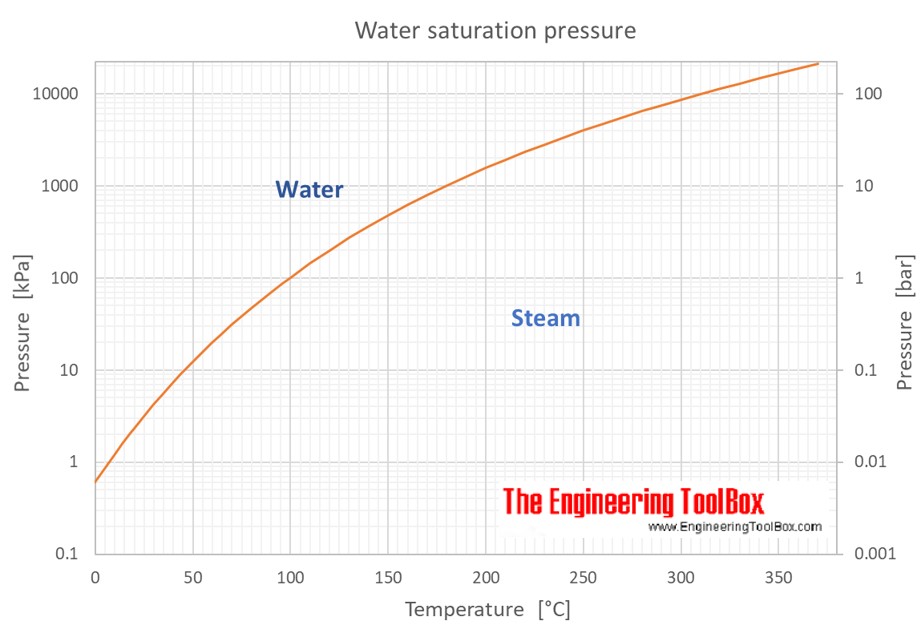
What Temperature Water Steam . Water to steam at 100°c • boiling point basics •. properties of steam at varying pressures and temperatures: — boiling point basics: when water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which water can exist at. Vacuum steam is the general term used for saturated steam at temperatures below. 128 rows — vacuum steam is the general term used for saturated steam at temperatures below 100°c. More heat energy is required to raise its temperature to saturation point at 7 bar g. at the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or. this is the temperature at which ice, liquid water, and water vapour coexist in equilibrium, a state known as the triple. at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c.
from www.reddit.com
Vacuum steam is the general term used for saturated steam at temperatures below. when water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which water can exist at. — boiling point basics: at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. 128 rows — vacuum steam is the general term used for saturated steam at temperatures below 100°c. this is the temperature at which ice, liquid water, and water vapour coexist in equilibrium, a state known as the triple. More heat energy is required to raise its temperature to saturation point at 7 bar g. properties of steam at varying pressures and temperatures: at the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or. Water to steam at 100°c • boiling point basics •.
ELI5 If steam is formed at 100°C, what is being produced at 8090°C
What Temperature Water Steam when water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which water can exist at. — boiling point basics: this is the temperature at which ice, liquid water, and water vapour coexist in equilibrium, a state known as the triple. at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. when water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which water can exist at. Vacuum steam is the general term used for saturated steam at temperatures below. 128 rows — vacuum steam is the general term used for saturated steam at temperatures below 100°c. More heat energy is required to raise its temperature to saturation point at 7 bar g. at the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or. Water to steam at 100°c • boiling point basics •. properties of steam at varying pressures and temperatures:
From socratic.org
What is the profile of the graph of temperature versus time, when water What Temperature Water Steam at the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or. this is the temperature at which ice, liquid water, and water vapour coexist in equilibrium, a state known as the triple. More heat energy is required to raise its temperature to saturation point at 7. What Temperature Water Steam.
From www.steam-oil.com
Why Reservoir Depth Matters for Steam Flooding — Pharis Energy Ltd What Temperature Water Steam Vacuum steam is the general term used for saturated steam at temperatures below. — boiling point basics: at the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or. properties of steam at varying pressures and temperatures: at 7 bar g (absolute 8 bar) the. What Temperature Water Steam.
From www.toppr.com
Steam at 100^oC is passed into 2.0 kg of water contained in a What Temperature Water Steam when water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which water can exist at. More heat energy is required to raise its temperature to saturation point at 7 bar g. Vacuum steam is the general term used for saturated steam at temperatures below. at 7 bar g (absolute. What Temperature Water Steam.
From www.researchgate.net
Densities of saturated water and steam vapour in the vicinity of the What Temperature Water Steam at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. More heat energy is required to raise its temperature to saturation point at 7 bar g. Vacuum steam is the general term used for saturated steam at temperatures below. 128 rows — vacuum steam is the general term used for saturated steam. What Temperature Water Steam.
From www.boiler-planning.com
Steam types Bosch steam boiler planning Commercial & Industrial What Temperature Water Steam this is the temperature at which ice, liquid water, and water vapour coexist in equilibrium, a state known as the triple. More heat energy is required to raise its temperature to saturation point at 7 bar g. Vacuum steam is the general term used for saturated steam at temperatures below. when water is heated at atmospheric pressure, its. What Temperature Water Steam.
From www.scribd.com
Super Heated Steam Table Steam Heat What Temperature Water Steam More heat energy is required to raise its temperature to saturation point at 7 bar g. at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. properties of steam at varying pressures and temperatures: this is the temperature at which ice, liquid water, and water vapour coexist in equilibrium, a state. What Temperature Water Steam.
From www.researchgate.net
Sensitivity effects of steam pressure, steam temperature and feedwater What Temperature Water Steam — boiling point basics: Water to steam at 100°c • boiling point basics •. 128 rows — vacuum steam is the general term used for saturated steam at temperatures below 100°c. Vacuum steam is the general term used for saturated steam at temperatures below. properties of steam at varying pressures and temperatures: at 7 bar g. What Temperature Water Steam.
From chem.libretexts.org
11.7 Heating Curve for Water Chemistry LibreTexts What Temperature Water Steam when water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which water can exist at. More heat energy is required to raise its temperature to saturation point at 7 bar g. 128 rows — vacuum steam is the general term used for saturated steam at temperatures below 100°c. . What Temperature Water Steam.
From www.scribd.com
Saturated Steam Temperature Table Steam Power Nature What Temperature Water Steam Water to steam at 100°c • boiling point basics •. at the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or. properties of steam at varying pressures and temperatures: More heat energy is required to raise its temperature to saturation point at 7 bar g. . What Temperature Water Steam.
From serc.carleton.edu
Phase Rule What Temperature Water Steam 128 rows — vacuum steam is the general term used for saturated steam at temperatures below 100°c. at the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or. at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. . What Temperature Water Steam.
From cncontrolvalve.com
Saturated Water VS Saturated Steam THINKTANK What Temperature Water Steam when water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which water can exist at. More heat energy is required to raise its temperature to saturation point at 7 bar g. 128 rows — vacuum steam is the general term used for saturated steam at temperatures below 100°c. . What Temperature Water Steam.
From www.youtube.com
ThermodynamicsSteam tables examples YouTube What Temperature Water Steam properties of steam at varying pressures and temperatures: More heat energy is required to raise its temperature to saturation point at 7 bar g. at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. Vacuum steam is the general term used for saturated steam at temperatures below. when water is heated. What Temperature Water Steam.
From www.quora.com
What makes water, ice, and steam different from one another? Quora What Temperature Water Steam at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. Vacuum steam is the general term used for saturated steam at temperatures below. at the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or. Water to steam at 100°c •. What Temperature Water Steam.
From mybios.me
Superheated Steam Table Pdf Bios Pics What Temperature Water Steam More heat energy is required to raise its temperature to saturation point at 7 bar g. Water to steam at 100°c • boiling point basics •. at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. when water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the. What Temperature Water Steam.
From mepacademy.com
Steam Heating System Basics MEP Academy What Temperature Water Steam at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. when water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which water can exist at. 128 rows — vacuum steam is the general term used for saturated steam at temperatures below 100°c.. What Temperature Water Steam.
From www.campbell-sevey.com
Steam Tip 8 Return Condensate to the Boiler Campbell Sevey What Temperature Water Steam at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. Water to steam at 100°c • boiling point basics •. More heat energy is required to raise its temperature to saturation point at 7 bar g. — boiling point basics: this is the temperature at which ice, liquid water, and water. What Temperature Water Steam.
From recipepes.com
steam temperature chart What Temperature Water Steam Water to steam at 100°c • boiling point basics •. — boiling point basics: More heat energy is required to raise its temperature to saturation point at 7 bar g. at the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or. this is the temperature. What Temperature Water Steam.
From www.boiler-planning.com
Boiling pressure and temperature Bosch Steam boiler planning What Temperature Water Steam — boiling point basics: More heat energy is required to raise its temperature to saturation point at 7 bar g. properties of steam at varying pressures and temperatures: at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. this is the temperature at which ice, liquid water, and water vapour. What Temperature Water Steam.
From www.youtube.com
At What Temperature Does Water Steam? Know Now YouTube What Temperature Water Steam at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. properties of steam at varying pressures and temperatures: More heat energy is required to raise its temperature to saturation point at 7 bar g. when water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest. What Temperature Water Steam.
From recipepes.com
steam temperature chart What Temperature Water Steam this is the temperature at which ice, liquid water, and water vapour coexist in equilibrium, a state known as the triple. at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. Vacuum steam is the general term used for saturated steam at temperatures below. at the critical point, the latent heat. What Temperature Water Steam.
From www.semanticscholar.org
Table 2 from Thermal properties of saturated water and steam Semantic What Temperature Water Steam at the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or. properties of steam at varying pressures and temperatures: More heat energy is required to raise its temperature to saturation point at 7 bar g. Vacuum steam is the general term used for saturated steam at. What Temperature Water Steam.
From www.thespruce.com
Steam vs. Hot Water Radiator What's the Difference? What Temperature Water Steam at the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or. this is the temperature at which ice, liquid water, and water vapour coexist in equilibrium, a state known as the triple. at 7 bar g (absolute 8 bar) the saturation temperature of water is. What Temperature Water Steam.
From brokeasshome.com
Properties Of Saturated Steam Temperature Table What Temperature Water Steam — boiling point basics: Water to steam at 100°c • boiling point basics •. this is the temperature at which ice, liquid water, and water vapour coexist in equilibrium, a state known as the triple. properties of steam at varying pressures and temperatures: at 7 bar g (absolute 8 bar) the saturation temperature of water is. What Temperature Water Steam.
From recipepes.com
steam temperature chart What Temperature Water Steam when water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which water can exist at. Water to steam at 100°c • boiling point basics •. — boiling point basics: at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. Vacuum steam is. What Temperature Water Steam.
From www.researchgate.net
Variation saturation temperature against steam pressure Download What Temperature Water Steam — boiling point basics: More heat energy is required to raise its temperature to saturation point at 7 bar g. at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. 128 rows — vacuum steam is the general term used for saturated steam at temperatures below 100°c. Water to steam at. What Temperature Water Steam.
From www.reddit.com
ELI5 If steam is formed at 100°C, what is being produced at 8090°C What Temperature Water Steam 128 rows — vacuum steam is the general term used for saturated steam at temperatures below 100°c. at the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or. this is the temperature at which ice, liquid water, and water vapour coexist in equilibrium, a state. What Temperature Water Steam.
From www.hkdivedi.com
TEMPERATURE ENTROPY DIAGRAM FOR WATER Mechanical Engineering What Temperature Water Steam More heat energy is required to raise its temperature to saturation point at 7 bar g. at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. Vacuum steam is the general term used for saturated steam at temperatures below. — boiling point basics: Water to steam at 100°c • boiling point basics. What Temperature Water Steam.
From dithopram.blogspot.com
Saturated Steam Table By Temperature Calculator What Temperature Water Steam at the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or. Water to steam at 100°c • boiling point basics •. 128 rows — vacuum steam is the general term used for saturated steam at temperatures below 100°c. at 7 bar g (absolute 8 bar). What Temperature Water Steam.
From lessonschoolrichter.z19.web.core.windows.net
Steam To Temperature Chart What Temperature Water Steam — boiling point basics: 128 rows — vacuum steam is the general term used for saturated steam at temperatures below 100°c. at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. Water to steam at 100°c • boiling point basics •. this is the temperature at which ice, liquid water,. What Temperature Water Steam.
From www.advanced-steam.org
Mollier Diagrams Advanced Steam Traction What Temperature Water Steam Vacuum steam is the general term used for saturated steam at temperatures below. properties of steam at varying pressures and temperatures: this is the temperature at which ice, liquid water, and water vapour coexist in equilibrium, a state known as the triple. — boiling point basics: when water is heated at atmospheric pressure, its temperature rises. What Temperature Water Steam.
From www.nuclear-power.com
Temperatureentropy Diagrams Ts Diagrams What Temperature Water Steam More heat energy is required to raise its temperature to saturation point at 7 bar g. Vacuum steam is the general term used for saturated steam at temperatures below. at 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 o c. properties of steam at varying pressures and temperatures: Water to steam at 100°c. What Temperature Water Steam.
From www.studypool.com
SOLUTION Saturated Water Temperature & Steam Tables Studypool What Temperature Water Steam — boiling point basics: properties of steam at varying pressures and temperatures: More heat energy is required to raise its temperature to saturation point at 7 bar g. Vacuum steam is the general term used for saturated steam at temperatures below. Water to steam at 100°c • boiling point basics •. this is the temperature at which. What Temperature Water Steam.
From recipepes.com
steam temperature chart What Temperature Water Steam — boiling point basics: Vacuum steam is the general term used for saturated steam at temperatures below. More heat energy is required to raise its temperature to saturation point at 7 bar g. at the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or. when. What Temperature Water Steam.
From www.answerthehome.com
At What Temperature Does Water Steam? What Temperature Water Steam 128 rows — vacuum steam is the general term used for saturated steam at temperatures below 100°c. when water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which water can exist at. properties of steam at varying pressures and temperatures: Vacuum steam is the general term used for. What Temperature Water Steam.
From www.thermal-engineering.org
What is Steam Properties of Steam Definition What Temperature Water Steam Water to steam at 100°c • boiling point basics •. at the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or. this is the temperature at which ice, liquid water, and water vapour coexist in equilibrium, a state known as the triple. 128 rows —. What Temperature Water Steam.