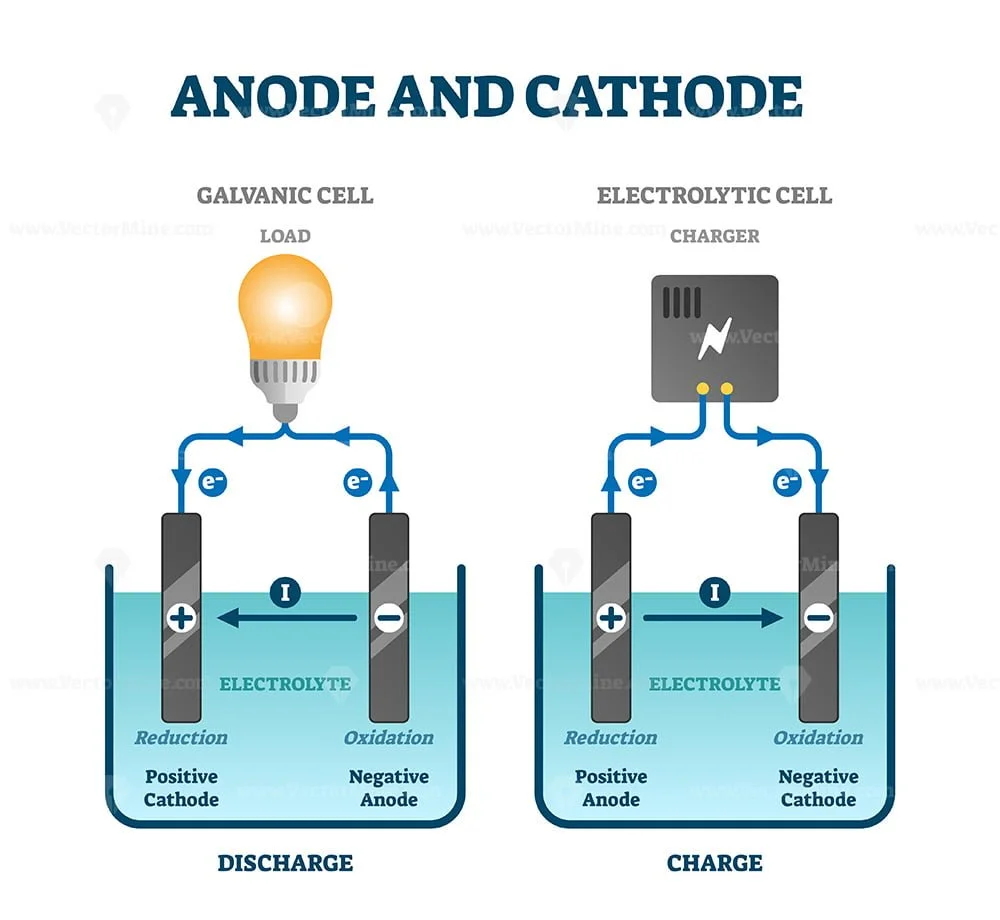
Electrochemistry Cathode Anode . The cathode is where reduction takes place and oxidation takes place at the anode. Electrons flow from the anode (electron provider or electron source) to the cathode. A cathode is an electrode from which the current exits a polarized electrical device while an anode is the electrode from which a current enters into a polarized electrical device. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. A commonly used language in electrochemistry is that of anode and cathode. The cathode is the electrode where. In a galvanic cell, it acts as the positive electrode since ions undergo reduction. The cathode serves as the site where reduction occurs, facilitating the gain of electrons. Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. The anode is defined as the electrode where oxidation occurs.
from vectormine.com
A commonly used language in electrochemistry is that of anode and cathode. Electrons flow from the anode (electron provider or electron source) to the cathode. The cathode is the electrode where. In a galvanic cell, it acts as the positive electrode since ions undergo reduction. Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. The cathode serves as the site where reduction occurs, facilitating the gain of electrons. The anode is defined as the electrode where oxidation occurs. A cathode is an electrode from which the current exits a polarized electrical device while an anode is the electrode from which a current enters into a polarized electrical device. Electrochemical cells have two conductive electrodes, called the anode and the cathode.
Anode and cathode scientific physics education diagram, vector
Electrochemistry Cathode Anode The cathode serves as the site where reduction occurs, facilitating the gain of electrons. Electrons flow from the anode (electron provider or electron source) to the cathode. Electrochemical cells have two conductive electrodes, called the anode and the cathode. The cathode is the electrode where. The cathode is where reduction takes place and oxidation takes place at the anode. The anode is defined as the electrode where oxidation occurs. Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. A cathode is an electrode from which the current exits a polarized electrical device while an anode is the electrode from which a current enters into a polarized electrical device. In a galvanic cell, it acts as the positive electrode since ions undergo reduction. Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. A commonly used language in electrochemistry is that of anode and cathode. The cathode serves as the site where reduction occurs, facilitating the gain of electrons.
From www.thoughtco.com
How to Define Anode and Cathode Electrochemistry Cathode Anode A cathode is an electrode from which the current exits a polarized electrical device while an anode is the electrode from which a current enters into a polarized electrical device. Electrochemical cells have two conductive electrodes, called the anode and the cathode. The cathode is the electrode where. Students are often confused that the sign convention of the anode and. Electrochemistry Cathode Anode.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Electrochemistry Cathode Anode The cathode is where reduction takes place and oxidation takes place at the anode. In a galvanic cell, it acts as the positive electrode since ions undergo reduction. Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. Electrochemical cells have two conductive electrodes, called the anode and the cathode. The anode is defined as the electrode where oxidation. Electrochemistry Cathode Anode.
From diagramlibrarynogg.z5.web.core.windows.net
Cathode In Electrochemical Cell Electrochemistry Cathode Anode Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. The cathode serves as the site where reduction occurs, facilitating the gain of electrons. A commonly used language. Electrochemistry Cathode Anode.
From www.researchgate.net
Electrochemical characterization of cathode and anode materials. CV Electrochemistry Cathode Anode Electrochemical cells have two conductive electrodes, called the anode and the cathode. Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. A commonly used language in electrochemistry is that of anode and cathode. The cathode serves as the site where reduction occurs, facilitating the gain of electrons. A cathode is. Electrochemistry Cathode Anode.
From slidetodoc.com
Basic Concepts of Electrochemical Cells Anode Cathode 1 Electrochemistry Cathode Anode A cathode is an electrode from which the current exits a polarized electrical device while an anode is the electrode from which a current enters into a polarized electrical device. The anode is defined as the electrode where oxidation occurs. Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. Electrochemical. Electrochemistry Cathode Anode.
From www.youtube.com
Cathode and Anode Quick differences and comparisons YouTube Electrochemistry Cathode Anode Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. The cathode serves as the site where reduction occurs, facilitating the gain of electrons. The cathode is where reduction takes place and oxidation takes place at the anode. A cathode is an electrode from which the current exits a polarized electrical. Electrochemistry Cathode Anode.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Electrochemistry Cathode Anode The cathode is the electrode where. The cathode is where reduction takes place and oxidation takes place at the anode. Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Electrons flow from the anode (electron provider or electron source) to the cathode. In a galvanic cell,. Electrochemistry Cathode Anode.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID3975392 Electrochemistry Cathode Anode Electrons flow from the anode (electron provider or electron source) to the cathode. A commonly used language in electrochemistry is that of anode and cathode. The cathode serves as the site where reduction occurs, facilitating the gain of electrons. In a galvanic cell, it acts as the positive electrode since ions undergo reduction. Electrochemical cells have two conductive electrodes, called. Electrochemistry Cathode Anode.
From www.youtube.com
Identify anode and cathode Electrochemistry Physical Chemistry Electrochemistry Cathode Anode Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. A commonly used language in electrochemistry is that of anode and cathode. Electrons flow from the anode (electron provider or electron source) to the cathode. A cathode is an electrode. Electrochemistry Cathode Anode.
From www.researchgate.net
Electrochemical cell with a flat M used A anode, C cathode Electrochemistry Cathode Anode Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. The cathode serves as the site where reduction occurs, facilitating the gain of electrons. The cathode is where reduction takes place and oxidation takes place at the anode. Electrons flow from the anode (electron provider or electron source) to the cathode. In a galvanic cell, it acts as the. Electrochemistry Cathode Anode.
From www.youtube.com
PCAT Electrochemistry Electrolysis, Anode, Cathode, & HalfReactions Electrochemistry Cathode Anode In a galvanic cell, it acts as the positive electrode since ions undergo reduction. Electrons flow from the anode (electron provider or electron source) to the cathode. The anode is defined as the electrode where oxidation occurs. The cathode is where reduction takes place and oxidation takes place at the anode. Students are often confused that the sign convention of. Electrochemistry Cathode Anode.
From herymad.weebly.com
Anode and cathode reaction in a h20 electrochemical cell herymad Electrochemistry Cathode Anode A cathode is an electrode from which the current exits a polarized electrical device while an anode is the electrode from which a current enters into a polarized electrical device. Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. The anode is defined as the electrode where oxidation occurs. In. Electrochemistry Cathode Anode.
From www.researchgate.net
Electrochemical characterizations of the anode and cathode under Electrochemistry Cathode Anode A cathode is an electrode from which the current exits a polarized electrical device while an anode is the electrode from which a current enters into a polarized electrical device. In a galvanic cell, it acts as the positive electrode since ions undergo reduction. Students are often confused that the sign convention of the anode and cathode switch for galvanic. Electrochemistry Cathode Anode.
From 2012books.lardbucket.org
Electrochemistry Electrochemistry Cathode Anode A commonly used language in electrochemistry is that of anode and cathode. The cathode is the electrode where. Electrochemical cells have two conductive electrodes, called the anode and the cathode. In a galvanic cell, it acts as the positive electrode since ions undergo reduction. Students are often confused that the sign convention of the anode and cathode switch for galvanic. Electrochemistry Cathode Anode.
From www.vedantu.com
Cathode and Anode Definition and Difference Between Anode and Cathode Electrochemistry Cathode Anode Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. In a galvanic cell, it acts as the positive electrode since ions undergo reduction. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Electrons flow from the anode (electron provider or electron source) to the cathode. A cathode is an electrode from which the current exits. Electrochemistry Cathode Anode.
From www.slideserve.com
PPT Topic Electrochemical Cells PowerPoint Presentation, free Electrochemistry Cathode Anode Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. A cathode is an electrode from which the current exits a polarized electrical device while an anode is the electrode from which a current enters into a polarized electrical device. Electrochemical cells have two conductive electrodes, called the anode and the cathode. The anode is defined as the electrode. Electrochemistry Cathode Anode.
From www.collegesearch.in
Cathode and Anode Definition, Examples, Differences CollegeSearch Electrochemistry Cathode Anode Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. Electrochemical cells have two conductive electrodes, called the anode and the cathode. The cathode is where reduction takes place and oxidation takes place at the anode. A commonly used language in electrochemistry is that of anode and cathode. The anode is defined as the electrode where oxidation occurs. Electrons. Electrochemistry Cathode Anode.
From diagramlibrarynogg.z5.web.core.windows.net
Picture Of Anode Electrolyte Cathode In Cell Electrochemistry Cathode Anode Electrochemical cells have two conductive electrodes, called the anode and the cathode. The cathode is the electrode where. A cathode is an electrode from which the current exits a polarized electrical device while an anode is the electrode from which a current enters into a polarized electrical device. The cathode serves as the site where reduction occurs, facilitating the gain. Electrochemistry Cathode Anode.
From vectormine.com
Anode and cathode scientific physics education diagram, vector Electrochemistry Cathode Anode A commonly used language in electrochemistry is that of anode and cathode. Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. The cathode serves as the site where reduction occurs, facilitating the gain of electrons. The anode is defined as the electrode where oxidation occurs. Electrons flow from the anode. Electrochemistry Cathode Anode.
From www.slideserve.com
PPT Topic Electrochemical Cells PowerPoint Presentation ID2281470 Electrochemistry Cathode Anode Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. The cathode is the electrode where. The cathode serves as the site where reduction occurs, facilitating the gain of electrons. In a galvanic cell, it acts as the positive electrode since ions undergo reduction. Electrons flow from the anode (electron provider. Electrochemistry Cathode Anode.
From enginelistmathew.z13.web.core.windows.net
Cathode In Electrochemical Cell Electrochemistry Cathode Anode Electrochemical cells have two conductive electrodes, called the anode and the cathode. The cathode is the electrode where. Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. Electrons flow from the anode (electron provider or electron source) to the cathode. A cathode is an electrode from which the current exits. Electrochemistry Cathode Anode.
From stock.adobe.com
Electrolytic cell infographic diagram with components including anode Electrochemistry Cathode Anode The cathode serves as the site where reduction occurs, facilitating the gain of electrons. The cathode is the electrode where. The anode is defined as the electrode where oxidation occurs. In a galvanic cell, it acts as the positive electrode since ions undergo reduction. Electrons flow from the anode (electron provider or electron source) to the cathode. Students are often. Electrochemistry Cathode Anode.
From learningcampusstall.z21.web.core.windows.net
Consider The Following Electrochemical Cell Electrochemistry Cathode Anode Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. The cathode serves as the site where reduction occurs, facilitating the gain of electrons. In a galvanic cell, it acts as the positive electrode since ions undergo reduction. The cathode is where reduction takes place and oxidation takes place at the anode. The anode is defined as the electrode. Electrochemistry Cathode Anode.
From 2012books.lardbucket.org
Electrochemistry Electrochemistry Cathode Anode The cathode is where reduction takes place and oxidation takes place at the anode. Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. The cathode serves as the site where reduction occurs, facilitating the gain of electrons. The cathode is the electrode where. A commonly used language in electrochemistry is that of anode and cathode. Students are often. Electrochemistry Cathode Anode.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID9623021 Electrochemistry Cathode Anode Electrons flow from the anode (electron provider or electron source) to the cathode. The cathode serves as the site where reduction occurs, facilitating the gain of electrons. The anode is defined as the electrode where oxidation occurs. The cathode is the electrode where. The cathode is where reduction takes place and oxidation takes place at the anode. A cathode is. Electrochemistry Cathode Anode.
From diagramlibrarypern.z21.web.core.windows.net
Cathode In Electrochemical Cell Electrochemistry Cathode Anode Electrochemical cells have two conductive electrodes, called the anode and the cathode. Electrons flow from the anode (electron provider or electron source) to the cathode. The anode is defined as the electrode where oxidation occurs. The cathode serves as the site where reduction occurs, facilitating the gain of electrons. The cathode is where reduction takes place and oxidation takes place. Electrochemistry Cathode Anode.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Electrochemistry Cathode Anode Electrons flow from the anode (electron provider or electron source) to the cathode. The cathode is the electrode where. In a galvanic cell, it acts as the positive electrode since ions undergo reduction. The anode is defined as the electrode where oxidation occurs. Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. The cathode serves as the site. Electrochemistry Cathode Anode.
From userpartfrieda.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram Electrochemistry Cathode Anode A commonly used language in electrochemistry is that of anode and cathode. The cathode is the electrode where. The cathode is where reduction takes place and oxidation takes place at the anode. The cathode serves as the site where reduction occurs, facilitating the gain of electrons. In a galvanic cell, it acts as the positive electrode since ions undergo reduction.. Electrochemistry Cathode Anode.
From hadassah-has-friedman.blogspot.com
Anode and Cathode in Electrolysis HadassahhasFriedman Electrochemistry Cathode Anode Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. In a galvanic cell, it acts as the positive electrode since ions undergo reduction. A cathode is an electrode from which the current exits a polarized electrical device while an anode is the electrode from which a current enters into a. Electrochemistry Cathode Anode.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Electrochemistry Cathode Anode The cathode serves as the site where reduction occurs, facilitating the gain of electrons. Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. Electrochemical cells have two conductive electrodes, called the anode and the cathode. In a galvanic cell,. Electrochemistry Cathode Anode.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5744606 Electrochemistry Cathode Anode Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. The cathode is where reduction takes place and oxidation takes place at the anode. A cathode is an electrode from which the current exits a polarized electrical device while an anode is the electrode from which a current enters into a polarized electrical device. A commonly used language in. Electrochemistry Cathode Anode.
From kayleyewabarr.blogspot.com
Anode and Cathode in Electrolysis KayleyewaBarr Electrochemistry Cathode Anode The cathode is the electrode where. A cathode is an electrode from which the current exits a polarized electrical device while an anode is the electrode from which a current enters into a polarized electrical device. The cathode is where reduction takes place and oxidation takes place at the anode. The anode is defined as the electrode where oxidation occurs.. Electrochemistry Cathode Anode.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrochemistry Cathode Anode Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. Electrons flow from the anode (electron provider or electron source) to the cathode. The cathode serves as the site where reduction occurs, facilitating the gain of electrons. The anode is defined as the electrode where oxidation occurs. In a galvanic cell,. Electrochemistry Cathode Anode.
From dokumen.tips
(PPTX) Unit 11 Redox and Electrochemistry Anode Cathode Electrochemistry Cathode Anode Students are often confused that the sign convention of the anode and cathode switch for galvanic and electrolytic cells. Electrochemical cells have two conductive electrodes, called the anode and the cathode. A cathode is an electrode from which the current exits a polarized electrical device while an anode is the electrode from which a current enters into a polarized electrical. Electrochemistry Cathode Anode.
From wiredbemerson.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram Electrochemistry Cathode Anode Electrochemical cells have two conductive electrodes, called the anode and the cathode. The cathode is the electrode where. Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. Electrons flow from the anode (electron provider or electron source) to the cathode. The anode is defined as the electrode where oxidation occurs. A cathode is an electrode from which the. Electrochemistry Cathode Anode.