What Is A Standard Formation Reaction . B) it is not the formation of a single. We can account for the energies lost and gained when reactants are consumed and products are formed. C(s) + o 2 (g) → co 2 (g) 3. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. A formation reaction is a reaction that produces one mole of a substance from its elements. 193 rows since the pressure of the standard formation reaction is fixed at 1 bar, the standard formation enthalpy or reaction heat is a. Realize that the reactions that. A formation reaction is the formation of one mole of a substance from its constituent elements. The above chemical reaction is the standard formation reaction for glucose. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 2) here are the reactions to be. This leads to the following version of hess’s law. We want the enthalpy for it.
from www.chegg.com
For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. B) it is not the formation of a single. The above chemical reaction is the standard formation reaction for glucose. A formation reaction is the formation of one mole of a substance from its constituent elements. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. This leads to the following version of hess’s law. Realize that the reactions that. We can account for the energies lost and gained when reactants are consumed and products are formed. 193 rows since the pressure of the standard formation reaction is fixed at 1 bar, the standard formation enthalpy or reaction heat is a. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free.
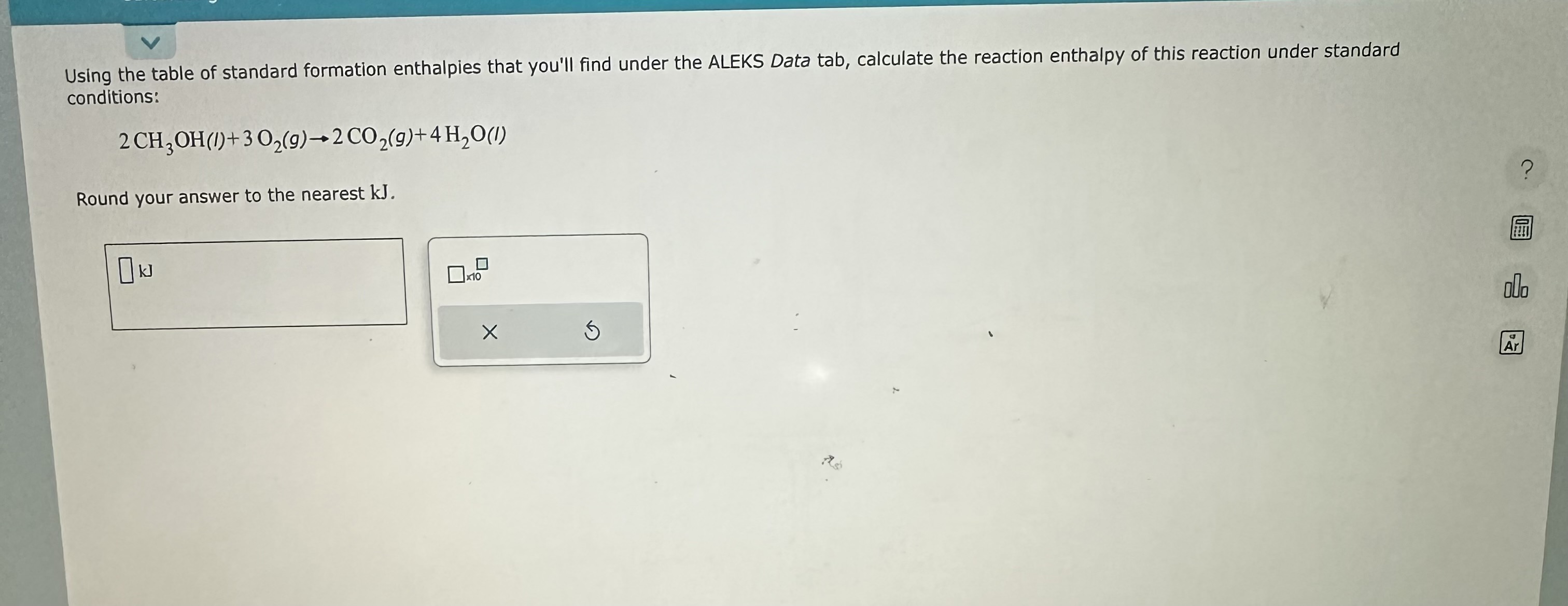
Solved Using the table of standard formation enthalpies that
What Is A Standard Formation Reaction A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. B) it is not the formation of a single. We can account for the energies lost and gained when reactants are consumed and products are formed. A formation reaction is the formation of one mole of a substance from its constituent elements. 193 rows since the pressure of the standard formation reaction is fixed at 1 bar, the standard formation enthalpy or reaction heat is a. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Realize that the reactions that. The above chemical reaction is the standard formation reaction for glucose. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. This leads to the following version of hess’s law. We want the enthalpy for it. 2) here are the reactions to be. A formation reaction is a reaction that produces one mole of a substance from its elements. C(s) + o 2 (g) → co 2 (g) 3.
From www.coursehero.com
[Solved] Using the table of standard formation enthalpies that you'll What Is A Standard Formation Reaction We can account for the energies lost and gained when reactants are consumed and products are formed. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows since the pressure of the standard formation reaction is fixed at 1 bar, the standard formation enthalpy. What Is A Standard Formation Reaction.
From www.chegg.com
Solved Write a balanced chemical equation for the standard What Is A Standard Formation Reaction This leads to the following version of hess’s law. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. We can account for the energies lost and gained when reactants are consumed and products are formed. A formation reaction is the formation of one mole of. What Is A Standard Formation Reaction.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube What Is A Standard Formation Reaction This leads to the following version of hess’s law. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free.. What Is A Standard Formation Reaction.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work What Is A Standard Formation Reaction 193 rows since the pressure of the standard formation reaction is fixed at 1 bar, the standard formation enthalpy or reaction heat is a. A formation reaction is the formation of one mole of a substance from its constituent elements. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. This leads. What Is A Standard Formation Reaction.
From www.chegg.com
Solved Using The Table Of Standard Formation Enthalpies T... What Is A Standard Formation Reaction 2) here are the reactions to be. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. B) it is not the formation of a single. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A formation. What Is A Standard Formation Reaction.
From people.chem.umass.edu
to Adobe GoLive 6 What Is A Standard Formation Reaction This leads to the following version of hess’s law. We want the enthalpy for it. C(s) + o 2 (g) → co 2 (g) 3. A formation reaction is the formation of one mole of a substance from its constituent elements. We can account for the energies lost and gained when reactants are consumed and products are formed. 193 rows. What Is A Standard Formation Reaction.
From www.youtube.com
Synthesis Reactions YouTube What Is A Standard Formation Reaction Realize that the reactions that. This leads to the following version of hess’s law. A formation reaction is the formation of one mole of a substance from its constituent elements. We want the enthalpy for it. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. 193 rows since the pressure of. What Is A Standard Formation Reaction.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper What Is A Standard Formation Reaction Realize that the reactions that. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. B) it is not the formation of a single. We want the enthalpy for it. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. What Is A Standard Formation Reaction.
From www.tessshebaylo.com
Balance The Following Chemical Equation And Calculate Standard Enthalpy What Is A Standard Formation Reaction A formation reaction is a reaction that produces one mole of a substance from its elements. We want the enthalpy for it. C(s) + o 2 (g) → co 2 (g) 3. The above chemical reaction is the standard formation reaction for glucose. 193 rows since the pressure of the standard formation reaction is fixed at 1 bar, the standard. What Is A Standard Formation Reaction.
From oneclass.com
OneClass Write a balanced chemical equation for the standard formation What Is A Standard Formation Reaction 193 rows since the pressure of the standard formation reaction is fixed at 1 bar, the standard formation enthalpy or reaction heat is a. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For any substance. What Is A Standard Formation Reaction.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table What Is A Standard Formation Reaction C(s) + o 2 (g) → co 2 (g) 3. A formation reaction is the formation of one mole of a substance from its constituent elements. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The above chemical reaction is the standard formation reaction for. What Is A Standard Formation Reaction.
From www.slideserve.com
PPT Formation Reactions PowerPoint Presentation, free download ID What Is A Standard Formation Reaction A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 2) here are the reactions to be. A formation reaction is the formation of one mole of a substance from its constituent elements. We can account for. What Is A Standard Formation Reaction.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax What Is A Standard Formation Reaction A formation reaction is the formation of one mole of a substance from its constituent elements. B) it is not the formation of a single. The above chemical reaction is the standard formation reaction for glucose. 193 rows since the pressure of the standard formation reaction is fixed at 1 bar, the standard formation enthalpy or reaction heat is a.. What Is A Standard Formation Reaction.
From aluminumhydroxidesaidan.blogspot.com
Aluminum Hydroxide Standard Formation Reaction Of Solid Aluminum Hydroxide What Is A Standard Formation Reaction A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. B) it is not the formation of a single. A formation reaction is a reaction that produces one mole of a substance from its elements. The above. What Is A Standard Formation Reaction.
From www.tessshebaylo.com
Write A Balanced Chemical Equation For The Standard Formation Reaction What Is A Standard Formation Reaction A formation reaction is the formation of one mole of a substance from its constituent elements. The above chemical reaction is the standard formation reaction for glucose. This leads to the following version of hess’s law. We can account for the energies lost and gained when reactants are consumed and products are formed. Realize that the reactions that. C(s) +. What Is A Standard Formation Reaction.
From www.slideserve.com
PPT Heat of Formation PowerPoint Presentation, free download ID3890043 What Is A Standard Formation Reaction A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Realize that the reactions that. B) it is not the formation of a single. For any substance at any particular temperature, we define the standard enthalpy of. What Is A Standard Formation Reaction.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps What Is A Standard Formation Reaction We can account for the energies lost and gained when reactants are consumed and products are formed. A formation reaction is a reaction that produces one mole of a substance from its elements. This leads to the following version of hess’s law. C(s) + o 2 (g) → co 2 (g) 3. The standard enthalpy of formation is a measure. What Is A Standard Formation Reaction.
From www.chegg.com
Solved Which of the following reactions is a standard What Is A Standard Formation Reaction 193 rows since the pressure of the standard formation reaction is fixed at 1 bar, the standard formation enthalpy or reaction heat is a. C(s) + o 2 (g) → co 2 (g) 3. 2) here are the reactions to be. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. B). What Is A Standard Formation Reaction.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies What Is A Standard Formation Reaction A formation reaction is a reaction that produces one mole of a substance from its elements. 193 rows since the pressure of the standard formation reaction is fixed at 1 bar, the standard formation enthalpy or reaction heat is a. Realize that the reactions that. This leads to the following version of hess’s law. B) it is not the formation. What Is A Standard Formation Reaction.
From quizzlistreplevies.z13.web.core.windows.net
How To Calculate The Heat Of Formation What Is A Standard Formation Reaction The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. This leads to the following version of hess’s law. C(s) + o 2 (g) → co 2 (g) 3. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change. What Is A Standard Formation Reaction.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics What Is A Standard Formation Reaction C(s) + o 2 (g) → co 2 (g) 3. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 2) here are the reactions to be. We want the enthalpy for it. We can account for. What Is A Standard Formation Reaction.
From www.youtube.com
Aleks Writing a standard formation reaction YouTube What Is A Standard Formation Reaction 2) here are the reactions to be. A formation reaction is a reaction that produces one mole of a substance from its elements. This leads to the following version of hess’s law. We can account for the energies lost and gained when reactants are consumed and products are formed. 193 rows since the pressure of the standard formation reaction is. What Is A Standard Formation Reaction.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry What Is A Standard Formation Reaction A formation reaction is the formation of one mole of a substance from its constituent elements. We can account for the energies lost and gained when reactants are consumed and products are formed. 2) here are the reactions to be. The above chemical reaction is the standard formation reaction for glucose. For any substance at any particular temperature, we define. What Is A Standard Formation Reaction.
From www.coursehero.com
[Solved] Please make sure it is balanced. Write a balanced chemical What Is A Standard Formation Reaction This leads to the following version of hess’s law. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free.. What Is A Standard Formation Reaction.
From www.youtube.com
ALEKS Writing a standard formation reaction YouTube What Is A Standard Formation Reaction We want the enthalpy for it. B) it is not the formation of a single. A formation reaction is the formation of one mole of a substance from its constituent elements. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance. What Is A Standard Formation Reaction.
From www.slideserve.com
PPT Formation Reactions PowerPoint Presentation, free download ID What Is A Standard Formation Reaction The above chemical reaction is the standard formation reaction for glucose. 2) here are the reactions to be. We can account for the energies lost and gained when reactants are consumed and products are formed. C(s) + o 2 (g) → co 2 (g) 3. This leads to the following version of hess’s law. The standard enthalpy of formation is. What Is A Standard Formation Reaction.
From www.coursehero.com
[Solved] Using the table of standard formation enthalpies that you'll What Is A Standard Formation Reaction We want the enthalpy for it. We can account for the energies lost and gained when reactants are consumed and products are formed. The above chemical reaction is the standard formation reaction for glucose. A formation reaction is a reaction that produces one mole of a substance from its elements. C(s) + o 2 (g) → co 2 (g) 3.. What Is A Standard Formation Reaction.
From www.chegg.com
Solved Writing a standard formation reaction write a What Is A Standard Formation Reaction C(s) + o 2 (g) → co 2 (g) 3. We want the enthalpy for it. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. B) it is not the formation of a single. The above chemical reaction is the standard formation reaction for glucose.. What Is A Standard Formation Reaction.
From www.chegg.com
Solved What is false about a standard formation reaction? What Is A Standard Formation Reaction 193 rows since the pressure of the standard formation reaction is fixed at 1 bar, the standard formation enthalpy or reaction heat is a. We can account for the energies lost and gained when reactants are consumed and products are formed. 2) here are the reactions to be. For any substance at any particular temperature, we define the standard enthalpy. What Is A Standard Formation Reaction.
From www.chegg.com
Solved Using the table of standard formation enthalpies that What Is A Standard Formation Reaction A formation reaction is the formation of one mole of a substance from its constituent elements. Realize that the reactions that. We want the enthalpy for it. 193 rows since the pressure of the standard formation reaction is fixed at 1 bar, the standard formation enthalpy or reaction heat is a. B) it is not the formation of a single.. What Is A Standard Formation Reaction.
From printableliblayette.z13.web.core.windows.net
Heat Of Formation Chart What Is A Standard Formation Reaction A formation reaction is a reaction that produces one mole of a substance from its elements. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A formation reaction is the formation of one mole of a. What Is A Standard Formation Reaction.
From www.chegg.com
Solved What is false about a standard formation reaction? What Is A Standard Formation Reaction We want the enthalpy for it. This leads to the following version of hess’s law. 193 rows since the pressure of the standard formation reaction is fixed at 1 bar, the standard formation enthalpy or reaction heat is a. 2) here are the reactions to be. A standard enthalpy of formation δ h f ° δ h f ° is. What Is A Standard Formation Reaction.
From printablelibmolines.z13.web.core.windows.net
How To Find Standard Enthalpy Of Reaction What Is A Standard Formation Reaction This leads to the following version of hess’s law. We want the enthalpy for it. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The above chemical reaction is the standard formation reaction for glucose. For any substance at any particular temperature, we define the. What Is A Standard Formation Reaction.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF What Is A Standard Formation Reaction This leads to the following version of hess’s law. C(s) + o 2 (g) → co 2 (g) 3. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A formation reaction is the formation of one mole of a substance from its constituent elements. We. What Is A Standard Formation Reaction.
From www.chegg.com
Solved Using the standard enthalpies of formation listed in What Is A Standard Formation Reaction B) it is not the formation of a single. 193 rows since the pressure of the standard formation reaction is fixed at 1 bar, the standard formation enthalpy or reaction heat is a. This leads to the following version of hess’s law. We can account for the energies lost and gained when reactants are consumed and products are formed. Realize. What Is A Standard Formation Reaction.