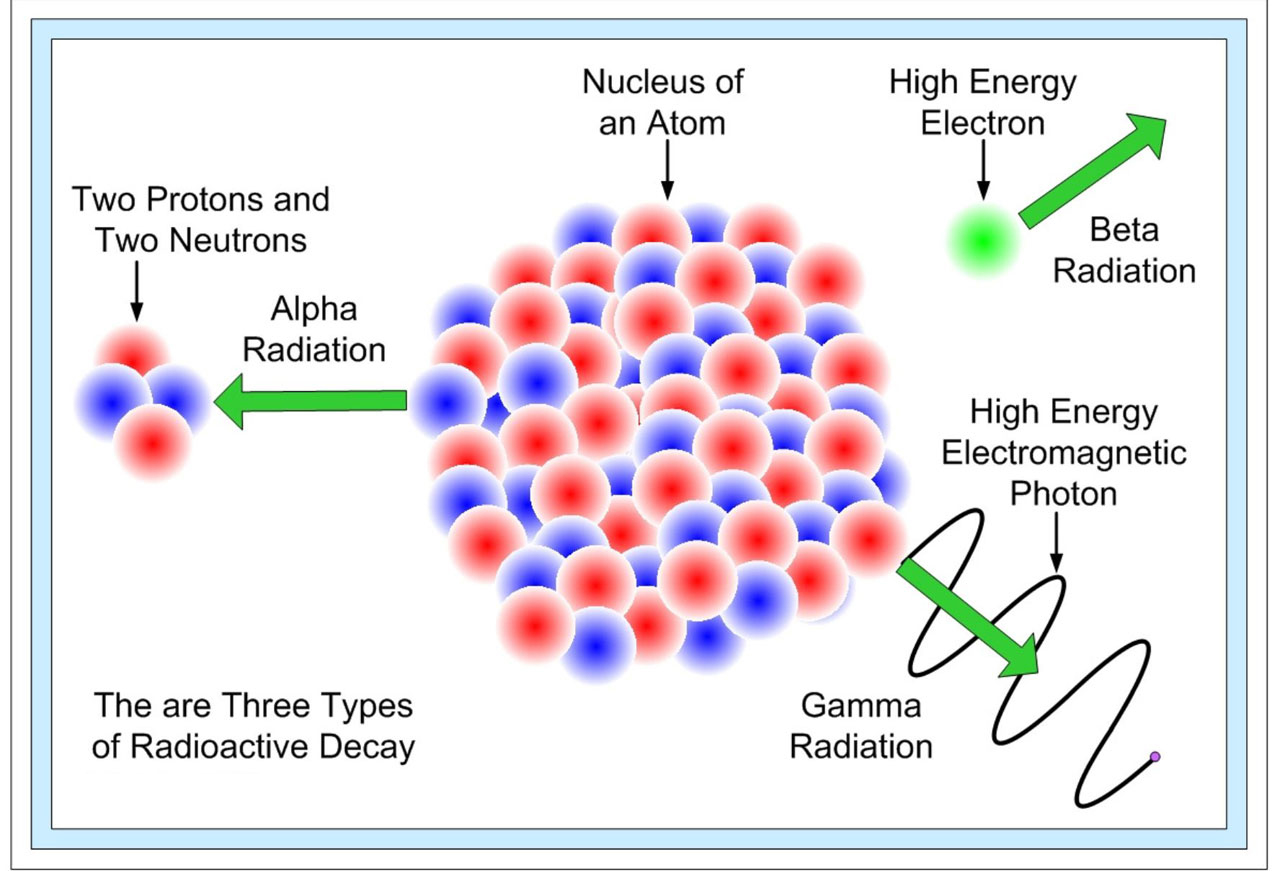
Why Are Radioisotopes Radioactive . Some forms of radiation are dangerous. Different isotopes of the same element have the same number of protons in their atomic nuclei but differing numbers of neutrons. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that is, its dpm;. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Exposure to radiation generally is. A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical. These are called radioactive isotopes. First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements.
from www.toppr.com
A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Some forms of radiation are dangerous. First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements. Exposure to radiation generally is. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that is, its dpm;. Different isotopes of the same element have the same number of protons in their atomic nuclei but differing numbers of neutrons. These are called radioactive isotopes.
Radioactivity Law of Radioactive Decay, Decay Rate, HalfMean Life, Q&A
Why Are Radioisotopes Radioactive These are called radioactive isotopes. The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that is, its dpm;. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Some forms of radiation are dangerous. Different isotopes of the same element have the same number of protons in their atomic nuclei but differing numbers of neutrons. Exposure to radiation generally is. A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical. These are called radioactive isotopes.
From ausearthed.blogspot.com
Radioactive Popcorn Why Are Radioisotopes Radioactive These are called radioactive isotopes. Some forms of radiation are dangerous. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements. Some isotopes are unstable and emit radiation in the form of particles and. Why Are Radioisotopes Radioactive.
From www.britannica.com
How Radioactive Isotopes are Used in Medicine Why Are Radioisotopes Radioactive Some forms of radiation are dangerous. The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that is, its dpm;. Different isotopes of the same element have the same number of protons in their atomic nuclei but differing numbers of neutrons. A radioactive isotope, also known as a. Why Are Radioisotopes Radioactive.
From www.moravek.com
How Radioisotopes Are Used in Medicine Today Why Are Radioisotopes Radioactive Exposure to radiation generally is. Different isotopes of the same element have the same number of protons in their atomic nuclei but differing numbers of neutrons. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. These are called radioactive isotopes. Some forms of radiation are dangerous. The total amount of radioisotope. Why Are Radioisotopes Radioactive.
From askthenurseexpert.com
Cancer Treatment Options Surgery, Radiation, And Chemotherapy Ask Why Are Radioisotopes Radioactive The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that is, its dpm;. Exposure to radiation generally is. Some forms of radiation are dangerous. First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements. Some isotopes are unstable and. Why Are Radioisotopes Radioactive.
From www.uvm.edu
Basics of Radioisotopes Why Are Radioisotopes Radioactive Exposure to radiation generally is. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that is, its dpm;. Radioactive isotopes, or radioisotopes, are species of chemical elements that. Why Are Radioisotopes Radioactive.
From www.geeksforgeeks.org
Radioactive Elements History, Examples, List, and Applications Why Are Radioisotopes Radioactive Some forms of radiation are dangerous. Different isotopes of the same element have the same number of protons in their atomic nuclei but differing numbers of neutrons. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. These are called radioactive isotopes. First, some radioactive isotopes form naturally on earth as. Why Are Radioisotopes Radioactive.
From www.slideserve.com
PPT What are Isotopes? PowerPoint Presentation, free download ID Why Are Radioisotopes Radioactive The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that is, its dpm;. A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical. Exposure to radiation generally is. Some isotopes are unstable and emit radiation in. Why Are Radioisotopes Radioactive.
From www.toppr.com
Radioactivity Law of Radioactive Decay, Decay Rate, HalfMean Life, Q&A Why Are Radioisotopes Radioactive Exposure to radiation generally is. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical. First, some radioactive isotopes form naturally on earth as a result of the decay of. Why Are Radioisotopes Radioactive.
From facts.net
16 Captivating Facts About Radioactive Isotope Why Are Radioisotopes Radioactive First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements. These are called radioactive isotopes. The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that is, its dpm;. Exposure to radiation generally is. Some isotopes are unstable and emit. Why Are Radioisotopes Radioactive.
From www.youtube.com
Uses of Radioisotope in Industry Nuclear Energy Science YouTube Why Are Radioisotopes Radioactive The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that is, its dpm;. Some forms of radiation are dangerous. Different isotopes of the same element have the same number of protons in their atomic nuclei but differing numbers of neutrons. First, some radioactive isotopes form naturally on. Why Are Radioisotopes Radioactive.
From sciabc.us
Why Are Certain Elements Radioactive? » Science ABC Why Are Radioisotopes Radioactive Different isotopes of the same element have the same number of protons in their atomic nuclei but differing numbers of neutrons. Exposure to radiation generally is. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. These are called radioactive isotopes. A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive. Why Are Radioisotopes Radioactive.
From www.mdpi.com
Molecules Free FullText Future Prospective of Radiopharmaceuticals Why Are Radioisotopes Radioactive First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements. Exposure to radiation generally is. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. These are called radioactive isotopes. Some isotopes are unstable and emit radiation in the form of particles and energy. Why Are Radioisotopes Radioactive.
From old.saednews.com
Radioactive Isotopes, Nuclear Diagnosis and Medical Progress saednews Why Are Radioisotopes Radioactive A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. The total amount of radioisotope present in a sample at any instant in time may be determined from its rate. Why Are Radioisotopes Radioactive.
From sciencenotes.org
Radioactivity and the Types of Radioactive Decay Why Are Radioisotopes Radioactive First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements. The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that is, its dpm;. Some isotopes are unstable and emit radiation in the form of particles and energy to form. Why Are Radioisotopes Radioactive.
From telegra.ph
Relative Vs Radioactive Dating Telegraph Why Are Radioisotopes Radioactive Some forms of radiation are dangerous. A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical. The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that is, its dpm;. Exposure to radiation generally is. Radioactive isotopes,. Why Are Radioisotopes Radioactive.
From www.foronuclear.org
What are radioisotopes? Foro Nuclear Why Are Radioisotopes Radioactive Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements. The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that. Why Are Radioisotopes Radioactive.
From www.dreamstime.com
Radioactivity. Closeup of Radioactive Atom Stock Vector Illustration Why Are Radioisotopes Radioactive Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. These are called radioactive isotopes. Some forms of radiation are dangerous. A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide,. Why Are Radioisotopes Radioactive.
From www.britannica.com
radioactive isotope Facts Britannica Why Are Radioisotopes Radioactive Exposure to radiation generally is. Different isotopes of the same element have the same number of protons in their atomic nuclei but differing numbers of neutrons. These are called radioactive isotopes. A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical. Radioactive isotopes, or radioisotopes, are species of chemical. Why Are Radioisotopes Radioactive.
From courses.lumenlearning.com
Uses of Radioisotopes Chemistry Why Are Radioisotopes Radioactive Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements. A radioactive isotope, also known. Why Are Radioisotopes Radioactive.
From www.researchgate.net
TYPICAL RADIOISOTOPES AND THEIR USES FOR IMAGING Download Table Why Are Radioisotopes Radioactive Exposure to radiation generally is. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. Some forms of radiation are dangerous. A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical. The total amount of radioisotope present in a sample at. Why Are Radioisotopes Radioactive.
From www.pinterest.com
Radioisotope Easy Science Easy science, Science facts, Stem for kids Why Are Radioisotopes Radioactive Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. Different isotopes of the same element have the same number of protons in their atomic nuclei but differing numbers of neutrons. Some forms of radiation are dangerous. Exposure to radiation generally is. Some isotopes are unstable and emit radiation in the form. Why Are Radioisotopes Radioactive.
From www.youtube.com
Uses of radioactive isotopes Chemistry YouTube Why Are Radioisotopes Radioactive A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical. These are called radioactive isotopes. Some forms of radiation are dangerous. The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that is, its dpm;. Radioactive isotopes,. Why Are Radioisotopes Radioactive.
From courses.lumenlearning.com
Radioactive Decay General Chemistry Why Are Radioisotopes Radioactive Some forms of radiation are dangerous. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Exposure to radiation generally is. The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that is, its dpm;. Radioactive isotopes, or radioisotopes,. Why Are Radioisotopes Radioactive.
From courses.lumenlearning.com
Uses of Radioisotopes Chemistry for Majors Why Are Radioisotopes Radioactive First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements. Exposure to radiation generally is. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the. Why Are Radioisotopes Radioactive.
From study.com
Making Predictions About Radioactive Nuclei & Decay Lesson Why Are Radioisotopes Radioactive A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical. Exposure to radiation generally is. First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay. Why Are Radioisotopes Radioactive.
From www.slideshare.net
Uses of radioisotopes Why Are Radioisotopes Radioactive First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Some forms of radiation are dangerous. Different isotopes of the same element have the same number of protons in their atomic nuclei but. Why Are Radioisotopes Radioactive.
From www.rtu.lv
Medical Radioisotopes Institute of Particle Physics and Accelerator Why Are Radioisotopes Radioactive A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical. Exposure to radiation generally is. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. The total amount of radioisotope present in a sample at any instant in time may be. Why Are Radioisotopes Radioactive.
From www.slideserve.com
PPT Chemistry Chapter 3 and 24 Atoms, Ions, Isotopes and Nuclear Why Are Radioisotopes Radioactive Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical. Some forms of radiation are dangerous. The total amount of radioisotope present in a sample at any instant in time may. Why Are Radioisotopes Radioactive.
From www.youtube.com
What are Radiopharmaceuticals Radioactive tracers? Introduction to Why Are Radioisotopes Radioactive Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. Different isotopes of the same element have the same number of protons in their atomic nuclei but differing numbers of neutrons. The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of. Why Are Radioisotopes Radioactive.
From www.tessshebaylo.com
Radioactive Decay Equation Chemistry Tessshebaylo Why Are Radioisotopes Radioactive Exposure to radiation generally is. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. Different isotopes of the same element have the same number of protons in their atomic nuclei but differing numbers of neutrons. Some forms of radiation are dangerous. Some isotopes are unstable and emit radiation in the form. Why Are Radioisotopes Radioactive.
From www.slideshare.net
Radioactive isotopes Why Are Radioisotopes Radioactive Different isotopes of the same element have the same number of protons in their atomic nuclei but differing numbers of neutrons. The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that is, its dpm;. First, some radioactive isotopes form naturally on earth as a result of the. Why Are Radioisotopes Radioactive.
From www.yaclass.in
Radiochemistry and its applications — lesson. Science State Board, Class 9. Why Are Radioisotopes Radioactive First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements. Exposure to radiation generally is. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the. Why Are Radioisotopes Radioactive.
From www.slideserve.com
PPT Radioactive Isotopes and Half Life PowerPoint Presentation, free Why Are Radioisotopes Radioactive Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements. These are called radioactive isotopes. Some forms of radiation are dangerous. A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide,. Why Are Radioisotopes Radioactive.
From www.thoughtco.com
A List of Radioactive Elements Why Are Radioisotopes Radioactive Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. Exposure to. Why Are Radioisotopes Radioactive.
From passeportmtletudiant.com
Explainer Radiation and radioactive decay Passeport Montreal Why Are Radioisotopes Radioactive These are called radioactive isotopes. Some forms of radiation are dangerous. Exposure to radiation generally is. First, some radioactive isotopes form naturally on earth as a result of the decay of primordial radioactive elements. Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms. Some isotopes are unstable and emit radiation in. Why Are Radioisotopes Radioactive.