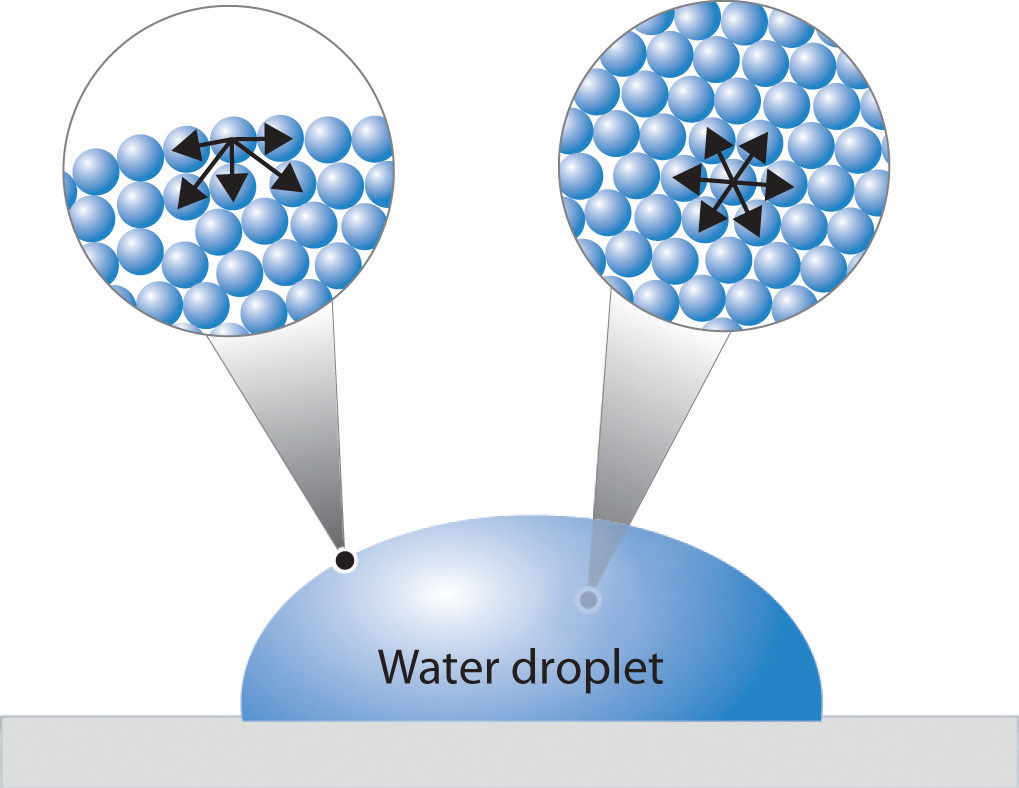
Explain Surface Tension Viscosity . Surface tension is the energy required to. Learn how cohesive and adhesive forces affect the behavior of liquids, such as surface tension, bubbles, droplets, and capillary action. Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. This is caused by the friction between molecules. Compared to viscosity, surface tension is a. Viscosity, on the other hand, is related to a liquid’s resistance to being deformed or moved. Surface tension refers to the cohesive forces between molecules at the surface of a liquid, causing it to behave like a stretched elastic. Learn how intermolecular forces affect the surface tension, viscosity, and capillary action of liquids. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its molecules and their size and shape. Learn the surface tension of water and the formula to find the surface tension of the given. The next time you are by a still body of water, take a. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water.
from chem.libretexts.org
Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Surface tension is the energy required to. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its molecules and their size and shape. Viscosity, on the other hand, is related to a liquid’s resistance to being deformed or moved. Learn how cohesive and adhesive forces affect the behavior of liquids, such as surface tension, bubbles, droplets, and capillary action. Compared to viscosity, surface tension is a. Learn how intermolecular forces affect the surface tension, viscosity, and capillary action of liquids. Learn the surface tension of water and the formula to find the surface tension of the given. The next time you are by a still body of water, take a. Surface tension refers to the cohesive forces between molecules at the surface of a liquid, causing it to behave like a stretched elastic.
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, and
Explain Surface Tension Viscosity Viscosity, on the other hand, is related to a liquid’s resistance to being deformed or moved. Surface tension refers to the cohesive forces between molecules at the surface of a liquid, causing it to behave like a stretched elastic. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Learn the surface tension of water and the formula to find the surface tension of the given. Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Surface tension is the energy required to. The next time you are by a still body of water, take a. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its molecules and their size and shape. Compared to viscosity, surface tension is a. Viscosity, on the other hand, is related to a liquid’s resistance to being deformed or moved. Learn how cohesive and adhesive forces affect the behavior of liquids, such as surface tension, bubbles, droplets, and capillary action. This is caused by the friction between molecules. Learn how intermolecular forces affect the surface tension, viscosity, and capillary action of liquids.
From www.youtube.com
Viscosity Surface Tension YouTube Explain Surface Tension Viscosity Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Learn how cohesive and adhesive forces affect the behavior of liquids, such as surface tension, bubbles, droplets, and capillary action. Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Learn the surface tension of water and the formula to. Explain Surface Tension Viscosity.
From www.scribd.com
Understanding Viscosity An InDepth Exploration of the Internal Explain Surface Tension Viscosity Viscosity, on the other hand, is related to a liquid’s resistance to being deformed or moved. The next time you are by a still body of water, take a. Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its. Explain Surface Tension Viscosity.
From dokumen.tips
(PPTX) Biomedical importance of surface tension & Viscosity DOKUMEN.TIPS Explain Surface Tension Viscosity Learn how intermolecular forces affect the surface tension, viscosity, and capillary action of liquids. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Surface tension refers to the cohesive forces between molecules at the surface of a liquid, causing it to behave like a stretched elastic. Compared to viscosity, surface tension is a.. Explain Surface Tension Viscosity.
From boardtopper.blogspot.com
Surface Tension & Viscosity All Study Guide at one Place! Explain Surface Tension Viscosity Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Learn how cohesive and adhesive forces affect the behavior of liquids, such as surface tension, bubbles, droplets, and capillary action. Compared to viscosity, surface tension is a. This is caused. Explain Surface Tension Viscosity.
From www.studypool.com
SOLUTION Surface tension and viscosity Studypool Explain Surface Tension Viscosity This is caused by the friction between molecules. Surface tension is the energy required to. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its molecules and their size and shape. Learn the surface tension of water and the formula to find the surface tension of the given. Surface tension refers to the. Explain Surface Tension Viscosity.
From www.researchgate.net
Variations of (a) the viscosity, (b) surface tension and (c) density Explain Surface Tension Viscosity Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its molecules and their size and shape. Learn the surface tension of water and the formula to find the surface tension of the given. Learn how cohesive and. Explain Surface Tension Viscosity.
From www.researchgate.net
Dependence of polymer solution viscosity (μ) and surface tension (γ) on Explain Surface Tension Viscosity The next time you are by a still body of water, take a. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its molecules and their size and shape. Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Surface tension is the elastic tendency of fluid surfaces that. Explain Surface Tension Viscosity.
From www.studypool.com
SOLUTION Surface tension viscosity handwritten notes english Studypool Explain Surface Tension Viscosity Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its molecules and their size and shape. Surface tension is the energy required to. Learn the surface tension of water and the formula to find the surface tension of the. Explain Surface Tension Viscosity.
From chem.libretexts.org
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, and Explain Surface Tension Viscosity This is caused by the friction between molecules. Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Learn the surface tension of water and the formula to find the surface tension of the given. Viscosity, on the other hand, is related to a liquid’s resistance to being deformed or moved. Surface tension is the energy. Explain Surface Tension Viscosity.
From www.youtube.com
12.3 Intermolecular Forces in Action Surface Tension & Viscosity YouTube Explain Surface Tension Viscosity Learn how intermolecular forces affect the surface tension, viscosity, and capillary action of liquids. The next time you are by a still body of water, take a. Compared to viscosity, surface tension is a. Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Learn how cohesive and adhesive forces affect the behavior of liquids, such. Explain Surface Tension Viscosity.
From www.studypool.com
SOLUTION Surface tension and viscosity 2 Studypool Explain Surface Tension Viscosity The next time you are by a still body of water, take a. This is caused by the friction between molecules. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its molecules and their size and shape. Compared to viscosity, surface tension is a. Viscosity, on the other hand, is related to a. Explain Surface Tension Viscosity.
From sophie-ksullivan.blogspot.com
Surface Tension and Viscosity Are Used to Describe Explain Surface Tension Viscosity Surface tension is the energy required to. Learn how cohesive and adhesive forces affect the behavior of liquids, such as surface tension, bubbles, droplets, and capillary action. Learn how intermolecular forces affect the surface tension, viscosity, and capillary action of liquids. This is caused by the friction between molecules. Compared to viscosity, surface tension is a. Viscosity, on the other. Explain Surface Tension Viscosity.
From www.studypool.com
SOLUTION Surface Tension & viscosity Physics Notes Studypool Explain Surface Tension Viscosity Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. The next time you are by a still body of water, take a. Learn the surface tension of water and the formula to find the surface tension of the given. Compared to viscosity, surface tension is a. Viscosity is a measure of a liquid's. Explain Surface Tension Viscosity.
From www.youtube.com
Surface Tension, Viscosity, & Melting Point YouTube Explain Surface Tension Viscosity Learn the surface tension of water and the formula to find the surface tension of the given. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its molecules and their size and shape. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Viscosity, on the other. Explain Surface Tension Viscosity.
From www.researchgate.net
Conductivity, dynamic viscosity, and surface tension of five Explain Surface Tension Viscosity The next time you are by a still body of water, take a. Viscosity, on the other hand, is related to a liquid’s resistance to being deformed or moved. This is caused by the friction between molecules. Learn the surface tension of water and the formula to find the surface tension of the given. Surface tension is the elastic tendency. Explain Surface Tension Viscosity.
From www.youtube.com
04 Ch 12 Viscosity and Surface Tension YouTube Explain Surface Tension Viscosity Learn how cohesive and adhesive forces affect the behavior of liquids, such as surface tension, bubbles, droplets, and capillary action. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its molecules and their size and shape. The next time you are by a still body of water, take a. Surface tension is the. Explain Surface Tension Viscosity.
From www.diplomageeks.com
Define capilirity, viscosity, coefficient of viscosity and surface Explain Surface Tension Viscosity This is caused by the friction between molecules. Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Learn the surface tension of water and the formula to find the surface tension of the given. Compared to viscosity, surface tension. Explain Surface Tension Viscosity.
From www.studypool.com
SOLUTION Surface tension and viscosity physics Studypool Explain Surface Tension Viscosity Learn how intermolecular forces affect the surface tension, viscosity, and capillary action of liquids. Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Viscosity, on the other hand, is related to a liquid’s resistance to being deformed or moved.. Explain Surface Tension Viscosity.
From www.studypool.com
SOLUTION Surface tension and viscosity physics Studypool Explain Surface Tension Viscosity Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Learn how intermolecular forces affect the surface tension, viscosity, and capillary action of liquids. Surface tension is the energy required to. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its molecules and their size and shape.. Explain Surface Tension Viscosity.
From www.studypool.com
SOLUTION Surface tension viscosity handwritten notes english Studypool Explain Surface Tension Viscosity Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Learn how intermolecular forces affect the surface tension, viscosity, and capillary action of liquids. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its molecules and their size and shape. The next time you are by a still body. Explain Surface Tension Viscosity.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru Explain Surface Tension Viscosity Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Surface tension is the energy required to. This is caused by the friction between molecules. Viscosity, on the other hand, is related to a liquid’s resistance to being deformed or moved. Learn the surface tension of water and the formula to find the surface tension of. Explain Surface Tension Viscosity.
From www.researchgate.net
The variation of solution viscosity and surface tension as a function Explain Surface Tension Viscosity Surface tension refers to the cohesive forces between molecules at the surface of a liquid, causing it to behave like a stretched elastic. Learn how cohesive and adhesive forces affect the behavior of liquids, such as surface tension, bubbles, droplets, and capillary action. Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Viscosity is a. Explain Surface Tension Viscosity.
From collegedunia.com
Viscosity Definition, Formula, Types & Examples Explain Surface Tension Viscosity Viscosity, on the other hand, is related to a liquid’s resistance to being deformed or moved. Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Viscosity is a measure of a liquid's resistance to flow, which depends on the. Explain Surface Tension Viscosity.
From www.researchgate.net
Variation of the viscosity and surface tension as a function of the Explain Surface Tension Viscosity Learn how cohesive and adhesive forces affect the behavior of liquids, such as surface tension, bubbles, droplets, and capillary action. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. The next time you are by a still body of water, take a. This is caused by the friction between molecules. Explain how the. Explain Surface Tension Viscosity.
From www.studypool.com
SOLUTION Surface tension and viscosity class 11th physics Studypool Explain Surface Tension Viscosity This is caused by the friction between molecules. Learn the surface tension of water and the formula to find the surface tension of the given. Viscosity, on the other hand, is related to a liquid’s resistance to being deformed or moved. Surface tension refers to the cohesive forces between molecules at the surface of a liquid, causing it to behave. Explain Surface Tension Viscosity.
From www.studypool.com
SOLUTION Fluid viscosity surface tension Studypool Explain Surface Tension Viscosity Explain how the surface tension and viscosity of a liquid relates to intermolecular forces. Surface tension refers to the cohesive forces between molecules at the surface of a liquid, causing it to behave like a stretched elastic. Learn how intermolecular forces affect the surface tension, viscosity, and capillary action of liquids. Viscosity, on the other hand, is related to a. Explain Surface Tension Viscosity.
From www.researchgate.net
The influence of vapor pressure, viscosity and surface tension on the Explain Surface Tension Viscosity The next time you are by a still body of water, take a. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. This is caused by the friction between molecules. Learn the surface tension of water and the formula to find the surface tension of the given. Learn how intermolecular forces affect the. Explain Surface Tension Viscosity.
From www.youtube.com
Surface Tension, Viscosity and Capillary Action YouTube Explain Surface Tension Viscosity Learn how cohesive and adhesive forces affect the behavior of liquids, such as surface tension, bubbles, droplets, and capillary action. Surface tension refers to the cohesive forces between molecules at the surface of a liquid, causing it to behave like a stretched elastic. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. This. Explain Surface Tension Viscosity.
From www.researchgate.net
The Surface Tension, Viscosity, Contact Angle of the inks on PVK layer Explain Surface Tension Viscosity Surface tension is the energy required to. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. The next time you are by a still body of water, take a. Learn how intermolecular forces affect the surface tension, viscosity, and capillary action of liquids. Viscosity is a measure of a liquid's resistance to flow,. Explain Surface Tension Viscosity.
From amt.copernicus.org
AMT The viscosity and surface tension of supercooled levitated Explain Surface Tension Viscosity The next time you are by a still body of water, take a. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Surface tension is the energy required to. Learn how intermolecular forces affect the surface tension, viscosity, and capillary action of liquids. Compared to viscosity, surface tension is a. Learn the surface. Explain Surface Tension Viscosity.
From www.studypool.com
SOLUTION Surface tension and viscosity 2 Studypool Explain Surface Tension Viscosity Viscosity, on the other hand, is related to a liquid’s resistance to being deformed or moved. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its molecules and their size and shape. Surface tension refers to the cohesive forces between molecules at the surface of a liquid, causing it to behave like a. Explain Surface Tension Viscosity.
From chem.libretexts.org
7.1 Surface Tension, Viscosity, and Capillary Action (Problems Explain Surface Tension Viscosity Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Learn the surface tension of water and the formula to find the surface tension of the given. This is caused by the friction between molecules. Learn how intermolecular forces affect the surface tension, viscosity, and capillary action of liquids. Surface tension refers to the. Explain Surface Tension Viscosity.
From www.researchgate.net
Surface tension and viscosity of various sample solutions Download Table Explain Surface Tension Viscosity Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Learn how cohesive and adhesive forces affect the behavior of liquids, such as surface tension, bubbles, droplets, and capillary action. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its molecules and their size and shape. Viscosity,. Explain Surface Tension Viscosity.
From www.studypool.com
SOLUTION Surface tension and viscosity class 11th physics Studypool Explain Surface Tension Viscosity Viscosity, on the other hand, is related to a liquid’s resistance to being deformed or moved. Learn how cohesive and adhesive forces affect the behavior of liquids, such as surface tension, bubbles, droplets, and capillary action. The next time you are by a still body of water, take a. Surface tension refers to the cohesive forces between molecules at the. Explain Surface Tension Viscosity.
From boardtopper.blogspot.com
Surface Tension & Viscosity All Study Guide at one Place! Explain Surface Tension Viscosity Surface tension refers to the cohesive forces between molecules at the surface of a liquid, causing it to behave like a stretched elastic. Viscosity is a measure of a liquid's resistance to flow, which depends on the imfs between its molecules and their size and shape. Learn how intermolecular forces affect the surface tension, viscosity, and capillary action of liquids.. Explain Surface Tension Viscosity.