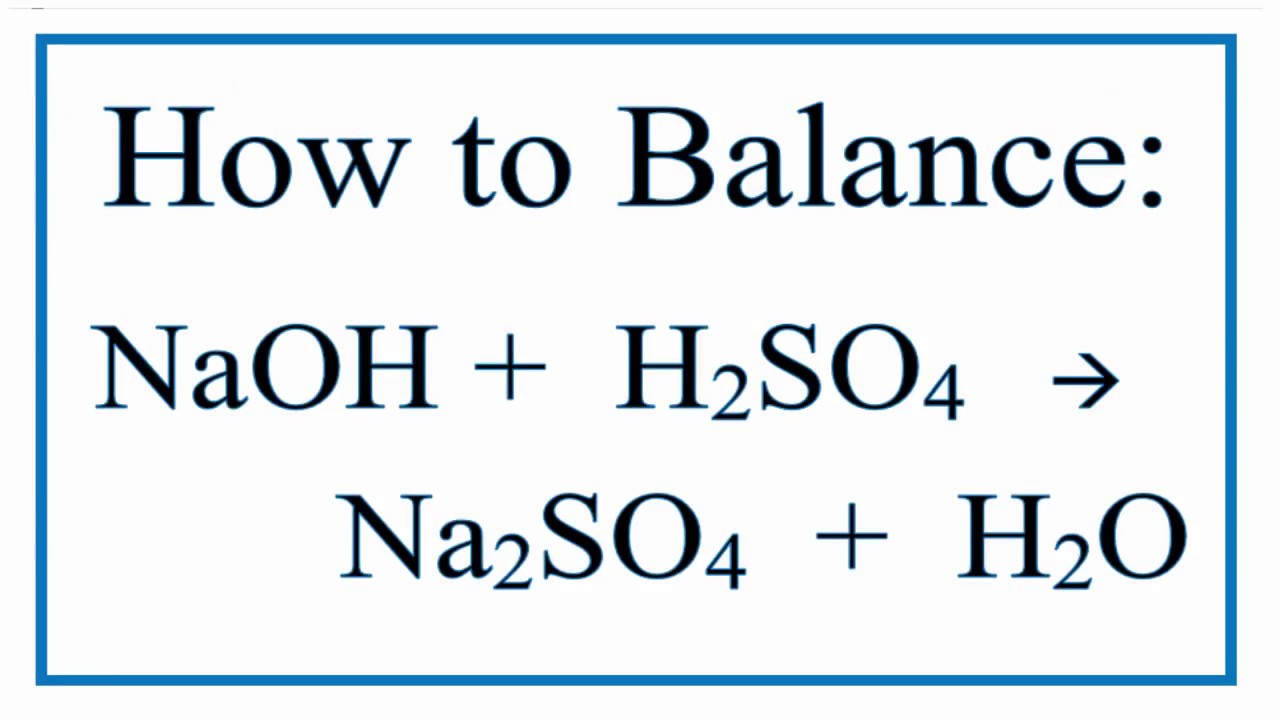
Titration H2So4 Naoh . Use the values for the averaged total volume of naoh added and the naoh concentration to calculate the moles of naoh used**.** 3**.** write and balance an equation to show. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. A $10~\mathrm{ml}$ sample of $\ce{h2so4}$ is removed and then titrated with $33.26~\mathrm{ml}$ of standard $0.2643\. Our titration calculator will help you never have to ask how do i calculate titrations? again. This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. Sulfuric acid can be neutralised by. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. If a third titration was required, average the two closest values. Sulfuric acid dissociates (ionises) in two stages: Sulfuric acid, h 2 so 4 is a strong diprotic acid. Average the values for the total volumes of naoh added. Use the values for the averaged total volume of naoh added and the.
from adelaideewawood.blogspot.com
$55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. Average the values for the total volumes of naoh added. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. Sulfuric acid dissociates (ionises) in two stages: Our titration calculator will help you never have to ask how do i calculate titrations? again. Sulfuric acid can be neutralised by. Use the values for the averaged total volume of naoh added and the. A $10~\mathrm{ml}$ sample of $\ce{h2so4}$ is removed and then titrated with $33.26~\mathrm{ml}$ of standard $0.2643\.
H2so4 Naoh Balanced Equation AdelaideewaWood
Titration H2So4 Naoh Sulfuric acid, h 2 so 4 is a strong diprotic acid. Average the values for the total volumes of naoh added. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. Sulfuric acid can be neutralised by. If a third titration was required, average the two closest values. Sulfuric acid dissociates (ionises) in two stages: A $10~\mathrm{ml}$ sample of $\ce{h2so4}$ is removed and then titrated with $33.26~\mathrm{ml}$ of standard $0.2643\. This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. Use the values for the averaged total volume of naoh added and the naoh concentration to calculate the moles of naoh used**.** 3**.** write and balance an equation to show. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Use the values for the averaged total volume of naoh added and the. Our titration calculator will help you never have to ask how do i calculate titrations? again. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. Sulfuric acid, h 2 so 4 is a strong diprotic acid.
From mungfali.com
The Titration Of 25 0 Ml Of An Unknown Concentration Of H2so4 Solution 699 Titration H2So4 Naoh If a third titration was required, average the two closest values. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. Sulfuric acid can be neutralised by. Sulfuric acid, h 2 so 4 is a strong diprotic acid. Sulfuric acid dissociates (ionises) in two stages: A $10~\mathrm{ml}$ sample of $\ce{h2so4}$ is removed and then titrated with $33.26~\mathrm{ml}$ of standard $0.2643\.. Titration H2So4 Naoh.
From giotpdccx.blob.core.windows.net
H2So4 Titration With Naoh Calculation at Selene Larsen blog Titration H2So4 Naoh A $10~\mathrm{ml}$ sample of $\ce{h2so4}$ is removed and then titrated with $33.26~\mathrm{ml}$ of standard $0.2643\. This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. Sulfuric acid, h 2 so 4 is a strong diprotic acid. Use the values for the averaged total volume of naoh added and the. Sulfuric acid can. Titration H2So4 Naoh.
From studylib.net
AcidBase Titration Answers, Sources of Error Titration H2So4 Naoh Average the values for the total volumes of naoh added. A $10~\mathrm{ml}$ sample of $\ce{h2so4}$ is removed and then titrated with $33.26~\mathrm{ml}$ of standard $0.2643\. Sulfuric acid, h 2 so 4 is a strong diprotic acid. Our titration calculator will help you never have to ask how do i calculate titrations? again. Firstly, clean the burette with distilled water (dw),. Titration H2So4 Naoh.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration H2So4 Naoh Use the values for the averaged total volume of naoh added and the. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. A $10~\mathrm{ml}$ sample of $\ce{h2so4}$ is removed and then titrated with $33.26~\mathrm{ml}$ of standard $0.2643\. Clean the pipette with dw, and rinse it with dilute solution of h 2 so. Titration H2So4 Naoh.
From studylib.net
Experiment (1) Standardization of sodium hydroxide NaOH solution Titration H2So4 Naoh Average the values for the total volumes of naoh added. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. A $10~\mathrm{ml}$ sample of $\ce{h2so4}$ is removed and then titrated with $33.26~\mathrm{ml}$ of standard $0.2643\. Our titration calculator will help you never have to ask how do i calculate titrations? again. This ensures that the interior portion of the burette. Titration H2So4 Naoh.
From mungfali.com
NaOH H2SO4 Na2SO4 H2O Titration H2So4 Naoh $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. Average the values for the total volumes of naoh added. Sulfuric acid, h 2 so 4 is a strong diprotic acid. Use the values for the averaged total volume of naoh added and the.. Titration H2So4 Naoh.
From www.numerade.com
SOLVED Write a balanced equation for the corresponding titration Titration H2So4 Naoh This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. Sulfuric acid dissociates (ionises) in two stages: Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. A $10~\mathrm{ml}$ sample of $\ce{h2so4}$ is removed and then titrated with $33.26~\mathrm{ml}$ of standard $0.2643\. Use the values for. Titration H2So4 Naoh.
From giotpdccx.blob.core.windows.net
H2So4 Titration With Naoh Calculation at Selene Larsen blog Titration H2So4 Naoh Use the values for the averaged total volume of naoh added and the. Sulfuric acid, h 2 so 4 is a strong diprotic acid. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. A $10~\mathrm{ml}$ sample of $\ce{h2so4}$ is removed and then. Titration H2So4 Naoh.
From www.numerade.com
SOLVED 'Determination the normality of a sodium hydroxide solution Titration H2So4 Naoh Our titration calculator will help you never have to ask how do i calculate titrations? again. Use the values for the averaged total volume of naoh added and the. This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. Firstly, clean the burette with distilled water (dw), and then rinse it with. Titration H2So4 Naoh.
From questions.kunduz.com
A titration experiment required 40.03 mL Physical Chemistry Titration H2So4 Naoh This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. Sulfuric acid, h 2 so 4 is a strong diprotic acid. Our titration calculator will help you never have to ask how do i calculate titrations? again. Sulfuric acid dissociates (ionises) in two stages: Use the values for the averaged total volume. Titration H2So4 Naoh.
From www.numerade.com
SOLVEDCalculate the volume in milliliters of a 1.420 M NaOH solution Titration H2So4 Naoh Use the values for the averaged total volume of naoh added and the. Sulfuric acid can be neutralised by. If a third titration was required, average the two closest values. Use the values for the averaged total volume of naoh added and the naoh concentration to calculate the moles of naoh used**.** 3**.** write and balance an equation to show.. Titration H2So4 Naoh.
From mavink.com
H2so4 Titration Curve Titration H2So4 Naoh Use the values for the averaged total volume of naoh added and the. Use the values for the averaged total volume of naoh added and the naoh concentration to calculate the moles of naoh used**.** 3**.** write and balance an equation to show. Average the values for the total volumes of naoh added. Firstly, clean the burette with distilled water. Titration H2So4 Naoh.
From www.solutioninn.com
[Solved] The student performs a second titration u SolutionInn Titration H2So4 Naoh A $10~\mathrm{ml}$ sample of $\ce{h2so4}$ is removed and then titrated with $33.26~\mathrm{ml}$ of standard $0.2643\. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Sulfuric acid can be neutralised by. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. Our titration calculator will help you never have to ask how do. Titration H2So4 Naoh.
From www.vrogue.co
Titration Practical And Calculation Naoh And Hcl Yout vrogue.co Titration H2So4 Naoh This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. Sulfuric acid, h 2 so 4 is a strong diprotic acid. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. Our titration calculator will help you never have to ask how do i calculate titrations? again. Firstly, clean the burette with. Titration H2So4 Naoh.
From giomzfpyz.blob.core.windows.net
Titration Of Naoh By H2So4 at Judith Phillips blog Titration H2So4 Naoh Sulfuric acid can be neutralised by. A $10~\mathrm{ml}$ sample of $\ce{h2so4}$ is removed and then titrated with $33.26~\mathrm{ml}$ of standard $0.2643\. Use the values for the averaged total volume of naoh added and the naoh concentration to calculate the moles of naoh used**.** 3**.** write and balance an equation to show. Clean the pipette with dw, and rinse it with. Titration H2So4 Naoh.
From www.chegg.com
Solved The curve shows the titration of H2SO3 with NaOH. The Titration H2So4 Naoh Sulfuric acid, h 2 so 4 is a strong diprotic acid. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Use the values for the averaged total volume of naoh added and the naoh concentration to calculate the moles of naoh used**.**. Titration H2So4 Naoh.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration H2So4 Naoh Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Average the values for the total volumes of naoh added. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. Our titration calculator will help you never have to ask how do i calculate titrations? again. If a third titration was required, average. Titration H2So4 Naoh.
From www.toppr.com
tion 11 Phenolphthalein is not a good indicator titrating NaOH against Titration H2So4 Naoh Average the values for the total volumes of naoh added. Sulfuric acid can be neutralised by. If a third titration was required, average the two closest values. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. Use the values for the averaged total volume of naoh added and the naoh concentration to calculate. Titration H2So4 Naoh.
From giomzfpyz.blob.core.windows.net
Titration Of Naoh By H2So4 at Judith Phillips blog Titration H2So4 Naoh Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Sulfuric acid dissociates (ionises) in two stages: Sulfuric acid, h 2 so 4 is a strong diprotic acid. If a third titration was required, average the two. Titration H2So4 Naoh.
From www.visionlearning.com
Acids and Bases I Math in Science Visionlearning Titration H2So4 Naoh Use the values for the averaged total volume of naoh added and the. Sulfuric acid dissociates (ionises) in two stages: Sulfuric acid can be neutralised by. Use the values for the averaged total volume of naoh added and the naoh concentration to calculate the moles of naoh used**.** 3**.** write and balance an equation to show. This ensures that the. Titration H2So4 Naoh.
From chemistry291.blogspot.com
H2SO4 + NaOH Sulfuric acid(H2SO4) and Sodium hydroxide(NaOH)What is Titration H2So4 Naoh Sulfuric acid can be neutralised by. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Sulfuric acid dissociates (ionises) in two stages: Use the values for the averaged total volume of naoh added and the. Sulfuric acid, h 2 so 4 is a strong diprotic acid. Clean the pipette with dw, and. Titration H2So4 Naoh.
From byjus.com
The graph of pH during the titration of NaOH and HCl Titration H2So4 Naoh Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Average the values for the total volumes of naoh added. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. Use the values for the averaged total volume of naoh added and the. If a third titration was required, average the two closest. Titration H2So4 Naoh.
From www.researchgate.net
Titration of 1 mL diluted bath (H2SO4/H3PO4) with 0.5 M NaOH in water Titration H2So4 Naoh Sulfuric acid, h 2 so 4 is a strong diprotic acid. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. A $10~\mathrm{ml}$ sample of $\ce{h2so4}$ is removed and then titrated with $33.26~\mathrm{ml}$ of standard $0.2643\. Average the values for the total volumes of naoh added. Sulfuric acid dissociates (ionises) in two stages:. Titration H2So4 Naoh.
From giomzfpyz.blob.core.windows.net
Titration Of Naoh By H2So4 at Judith Phillips blog Titration H2So4 Naoh Our titration calculator will help you never have to ask how do i calculate titrations? again. Sulfuric acid, h 2 so 4 is a strong diprotic acid. This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. If a third titration was required, average the two closest values. Use the values for. Titration H2So4 Naoh.
From www.youtube.com
Neutralisation Reaction Sulfuric Acid and Sodium Hydroxide Balancing Titration H2So4 Naoh Average the values for the total volumes of naoh added. If a third titration was required, average the two closest values. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Sulfuric acid can be neutralised by. Sulfuric acid, h 2 so 4 is a strong diprotic acid. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used. Titration H2So4 Naoh.
From www.numerade.com
SOLVED Phenolphthalein is not a good indicator for titrating NaOH Titration H2So4 Naoh Sulfuric acid can be neutralised by. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. If a third titration was required, average the two. Titration H2So4 Naoh.
From www.numerade.com
SOLVED Question 6 (1 point) A titration experiment required 10.25 mL Titration H2So4 Naoh Our titration calculator will help you never have to ask how do i calculate titrations? again. Sulfuric acid can be neutralised by. Use the values for the averaged total volume of naoh added and the naoh concentration to calculate the moles of naoh used**.** 3**.** write and balance an equation to show. Firstly, clean the burette with distilled water (dw),. Titration H2So4 Naoh.
From www.scribd.com
Acid Base Titration Lab H2so4 + Naoh AP Chem 2 Titration Chemistry Titration H2So4 Naoh Sulfuric acid, h 2 so 4 is a strong diprotic acid. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. Use the values for the averaged total volume of naoh added and the. If a third. Titration H2So4 Naoh.
From www.numerade.com
SOLVED In titrating 0.20 M sulfuric acid, H2SO4, with 0.4M NaOH at 25∘ Titration H2So4 Naoh Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. A $10~\mathrm{ml}$ sample of $\ce{h2so4}$ is removed and then titrated with $33.26~\mathrm{ml}$ of standard $0.2643\. If a third titration was required, average the two closest values. Sulfuric acid, h 2 so 4 is a strong diprotic acid. Use the values for the averaged. Titration H2So4 Naoh.
From adelaideewawood.blogspot.com
H2so4 Naoh Balanced Equation AdelaideewaWood Titration H2So4 Naoh Use the values for the averaged total volume of naoh added and the naoh concentration to calculate the moles of naoh used**.** 3**.** write and balance an equation to show. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. This ensures that the interior portion of the burette is coated with a. Titration H2So4 Naoh.
From www.numerade.com
SOLVED 'Question In SHN NaOH which S) H2SO4 3 points) + HCI Titration H2So4 Naoh Sulfuric acid can be neutralised by. This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. If a third titration was required, average the two closest values. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$. Titration H2So4 Naoh.
From www.chegg.com
Solved Use the following titration curve for the first four Titration H2So4 Naoh Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Sulfuric acid dissociates (ionises) in two stages: Our titration calculator will help you never have to ask how do i calculate titrations? again. Sulfuric acid can be neutralised by. Average the values for the total volumes of naoh added. Use the values for. Titration H2So4 Naoh.
From brainly.com
A solution of sulfuric acid is titrated with a solution of sodium Titration H2So4 Naoh Our titration calculator will help you never have to ask how do i calculate titrations? again. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. If a third titration was required, average the two closest values. Firstly, clean the burette with distilled water. Titration H2So4 Naoh.
From www.numerade.com
SOLVED a titration was performed on a 25.0 mL sample of H2SO4 using 17 Titration H2So4 Naoh Sulfuric acid dissociates (ionises) in two stages: Sulfuric acid, h 2 so 4 is a strong diprotic acid. This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium. Titration H2So4 Naoh.
From www.coursehero.com
[Solved] H2SO4 + NaOH > Na2SO4 + H20 Left H=3 > H= 2 S=1 Titration H2So4 Naoh Our titration calculator will help you never have to ask how do i calculate titrations? again. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. $55.0~\mathrm{ml}$ of $0.250~\mathrm{m}~\ce{naoh}$ is used to titrate $35.0~\mathrm{ml}$ of $\ce{h2so4}$. Sulfuric acid dissociates (ionises) in two stages: A $10~\mathrm{ml}$ sample of $\ce{h2so4}$ is removed and then titrated. Titration H2So4 Naoh.