Low Surface Tension Examples . Water has a surface tension of 0.07275. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. When a water droplet forms, it tends to take on a spherical shape due to surface tension. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The shape of the drops is caused by the surface tension of the water. The water molecules on the surface of the droplet. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the.
from www.geeksforgeeks.org
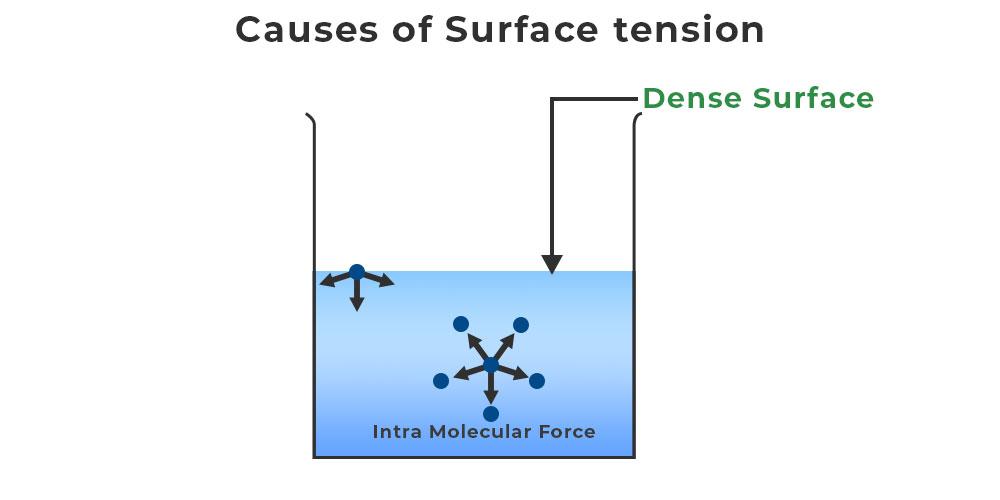
Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). When a water droplet forms, it tends to take on a spherical shape due to surface tension. The shape of the drops is caused by the surface tension of the water. The water molecules on the surface of the droplet. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. Water has a surface tension of 0.07275.
Surface Tension Definition, Formula, Causes, Examples, and FAQs
Low Surface Tension Examples Water has a surface tension of 0.07275. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The shape of the drops is caused by the surface tension of the water. When a water droplet forms, it tends to take on a spherical shape due to surface tension. Water has a surface tension of 0.07275. The water molecules on the surface of the droplet. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Low Surface Tension Examples Water has a surface tension of 0.07275. The shape of the drops is caused by the surface tension of the water. The water molecules on the surface of the droplet. When a water droplet forms, it tends to take on a spherical shape due to surface tension. The only reason the drop of water isn't completely spherical is that the. Low Surface Tension Examples.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3227401 Low Surface Tension Examples Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. The shape of the drops is caused by the surface tension of the water. Water has a surface tension of 0.07275. When a. Low Surface Tension Examples.
From www.biolinscientific.com
3 ways to measure surface tension Low Surface Tension Examples The water molecules on the surface of the droplet. Water has a surface tension of 0.07275. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. Surface tension may. Low Surface Tension Examples.
From proper-cooking.info
Surface Tension Examples In Nature Low Surface Tension Examples Water has a surface tension of 0.07275. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. When a water droplet forms, it tends to take on a spherical shape due to surface tension. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on. Low Surface Tension Examples.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Low Surface Tension Examples The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. “surface tension. Low Surface Tension Examples.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Low Surface Tension Examples Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. When a water droplet forms, it tends to take on a spherical shape due to surface tension. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. Surface tension may be expressed,. Low Surface Tension Examples.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson Low Surface Tension Examples Water has a surface tension of 0.07275. The shape of the drops is caused by the surface tension of the water. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). When a water. Low Surface Tension Examples.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Low Surface Tension Examples The shape of the drops is caused by the surface tension of the water. Water has a surface tension of 0.07275. When a water droplet forms, it tends to take on a spherical shape due to surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in. Low Surface Tension Examples.
From civilquery.com
What is Surface Tension Definition, Examples and Tests Civil Query Low Surface Tension Examples When a water droplet forms, it tends to take on a spherical shape due to surface tension. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. The water molecules on the surface of the droplet. The shape of the drops is caused by the surface tension of the water. Surface tension may. Low Surface Tension Examples.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Low Surface Tension Examples The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. Water has a surface tension of 0.07275. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. “surface tension is the tension of a liquid’s surface film caused by the bulk of the. Low Surface Tension Examples.
From www.researchgate.net
The role of a low surface tension liquid on the droplet wetting state Low Surface Tension Examples The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. Water has a surface tension of 0.07275. When using a water dropper, the water does not flow in a continuous stream, but rather. Low Surface Tension Examples.
From www.slideserve.com
PPT FLUID PROPERTIES Chapter 2 PowerPoint Presentation, free download Low Surface Tension Examples The shape of the drops is caused by the surface tension of the water. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. When a water droplet forms, it tends to take on a spherical shape due to. Low Surface Tension Examples.
From www.vrogue.co
A Variation Of The Surface Tension And Viscosity Of T vrogue.co Low Surface Tension Examples The water molecules on the surface of the droplet. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface. Low Surface Tension Examples.
From www.slideserve.com
PPT Ch. 2 States of Matter PowerPoint Presentation, free download Low Surface Tension Examples Water has a surface tension of 0.07275. The shape of the drops is caused by the surface tension of the water. When a water droplet forms, it tends to take on a spherical shape due to surface tension. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. The. Low Surface Tension Examples.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Low Surface Tension Examples Surface tension may be expressed, therefore, in units of energy per unit area (square metres). When a water droplet forms, it tends to take on a spherical shape due to surface tension. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. The water molecules on the surface of the droplet. “surface tension. Low Surface Tension Examples.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Low Surface Tension Examples The water molecules on the surface of the droplet. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. When a water droplet forms, it tends to take on a spherical shape due to surface tension. Surface tension may be expressed, therefore, in units of energy per unit area (square. Low Surface Tension Examples.
From www.youtube.com
Surface Tension of Water Explained YouTube Low Surface Tension Examples The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. Water has a surface tension of 0.07275. The shape of the drops is caused by the surface tension of the water. When using. Low Surface Tension Examples.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Low Surface Tension Examples Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. Water has a surface tension of 0.07275. When a water droplet forms, it tends to take on a spherical shape due to surface tension. The shape of the drops is caused by the surface tension of the water. “surface tension is the tension. Low Surface Tension Examples.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Low Surface Tension Examples Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. When a water droplet forms, it tends to take on a spherical shape due to surface tension. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. The shape of the drops is. Low Surface Tension Examples.
From eduinput.com
Surface TensionDefinition, Examples, Causes, And Measurement Low Surface Tension Examples Surface tension may be expressed, therefore, in units of energy per unit area (square metres). When a water droplet forms, it tends to take on a spherical shape due to surface tension. Water has a surface tension of 0.07275. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. When. Low Surface Tension Examples.
From byjus.com
Explain the surface tension phenomenon with examples. Low Surface Tension Examples The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The water molecules on the surface of the droplet.. Low Surface Tension Examples.
From www.adda247.com
Surface Tension Definition, Formula, Examples Low Surface Tension Examples When a water droplet forms, it tends to take on a spherical shape due to surface tension. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. Surface tension may be expressed, therefore,. Low Surface Tension Examples.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Low Surface Tension Examples When a water droplet forms, it tends to take on a spherical shape due to surface tension. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. Liquids with high surface tension exhibit significant resistance. Low Surface Tension Examples.
From sciencing.com
What Is the Difference Between High & Low Surface Tension? Sciencing Low Surface Tension Examples When a water droplet forms, it tends to take on a spherical shape due to surface tension. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. Surface tension may be expressed, therefore,. Low Surface Tension Examples.
From slidetodoc.com
Lecture 14 Taken in part from Chapters 13 Low Surface Tension Examples “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The water molecules on the surface of the droplet. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it.. Low Surface Tension Examples.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free Low Surface Tension Examples Surface tension may be expressed, therefore, in units of energy per unit area (square metres). The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. When a water droplet forms, it tends to take on a spherical shape due to surface tension. When using a water dropper, the water does. Low Surface Tension Examples.
From www.slideserve.com
PPT Chapter 5 Liquids and Solids PowerPoint Presentation, free Low Surface Tension Examples The water molecules on the surface of the droplet. Water has a surface tension of 0.07275. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. The shape of the drops is caused by the surface tension of the water. The only reason the drop of water isn't completely. Low Surface Tension Examples.
From www.vrogue.co
Surface Tension Definition Examples Causes And Measur vrogue.co Low Surface Tension Examples When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The shape of the drops is caused by. Low Surface Tension Examples.
From schematicpartebriose.z14.web.core.windows.net
Surface Tension Simple Explanation Low Surface Tension Examples Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. Water has a surface tension of 0.07275. The shape of the drops is caused by the surface tension of the water. Surface. Low Surface Tension Examples.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Low Surface Tension Examples Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). When using a water dropper, the water does. Low Surface Tension Examples.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension Low Surface Tension Examples Water has a surface tension of 0.07275. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. When a water droplet forms, it tends to take on a spherical. Low Surface Tension Examples.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint Low Surface Tension Examples Water has a surface tension of 0.07275. The water molecules on the surface of the droplet. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). When a water droplet forms, it tends to. Low Surface Tension Examples.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Low Surface Tension Examples Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. The shape of the drops is caused by the surface tension of the water. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. The water molecules on the surface of the. Low Surface Tension Examples.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit Low Surface Tension Examples When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. When a water droplet forms, it tends to take on a spherical shape due to surface tension. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. The shape. Low Surface Tension Examples.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Low Surface Tension Examples Surface tension may be expressed, therefore, in units of energy per unit area (square metres). The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. Liquids with high surface. Low Surface Tension Examples.