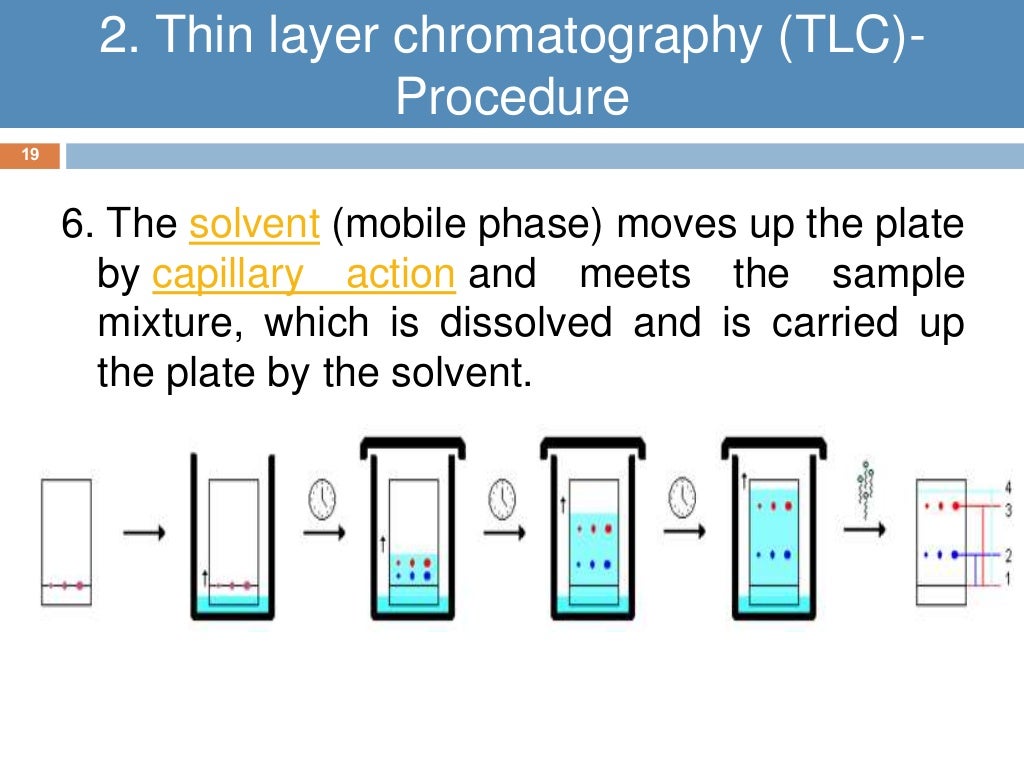
What Is The Solvent In Tlc . When the spot of mixture is dry, the plate is stood in a shallow layer of solvent in a covered beaker. As a rule of thumb, a concentration of 1%. If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate, or methylene chloride. The optimal concentration for tlc is typically determined empirically, but a good place to. What solvents do you use in thin layer chromatography? In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. Using a capillary tube, a. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : The typical eluent for tlc is a mixture of an apolar solvent (typically. Ideally the vials will have a lid to minimize vapors and preserve the samples if tipped over (figure 2.23a). It is important that the solvent level is below the line with the spot on it.
from es.slideshare.net
As a rule of thumb, a concentration of 1%. Ideally the vials will have a lid to minimize vapors and preserve the samples if tipped over (figure 2.23a). When the spot of mixture is dry, the plate is stood in a shallow layer of solvent in a covered beaker. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : The typical eluent for tlc is a mixture of an apolar solvent (typically. What solvents do you use in thin layer chromatography? In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. The optimal concentration for tlc is typically determined empirically, but a good place to. It is important that the solvent level is below the line with the spot on it. If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate, or methylene chloride.
Chromatography (paper chromatography and tlc)
What Is The Solvent In Tlc It is important that the solvent level is below the line with the spot on it. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : Using a capillary tube, a. It is important that the solvent level is below the line with the spot on it. As a rule of thumb, a concentration of 1%. Ideally the vials will have a lid to minimize vapors and preserve the samples if tipped over (figure 2.23a). When the spot of mixture is dry, the plate is stood in a shallow layer of solvent in a covered beaker. The typical eluent for tlc is a mixture of an apolar solvent (typically. If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate, or methylene chloride. What solvents do you use in thin layer chromatography? In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. The optimal concentration for tlc is typically determined empirically, but a good place to.
From www.numerade.com
SOLVED What does the above TLC experiment tell you about the effect of What Is The Solvent In Tlc Using a capillary tube, a. When the spot of mixture is dry, the plate is stood in a shallow layer of solvent in a covered beaker. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. What solvents do you use. What Is The Solvent In Tlc.
From www.biotage.com
Why is Solvent Evaluation by TLC Important for Good Flash What Is The Solvent In Tlc A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : It is important that the solvent level is below the line with the spot on it. Using a capillary tube, a. The optimal concentration for tlc is typically determined empirically, but a good place to. Ideally the vials will have a lid to. What Is The Solvent In Tlc.
From hxeemavye.blob.core.windows.net
Tlc Solvent Guide at David Richards blog What Is The Solvent In Tlc It is important that the solvent level is below the line with the spot on it. As a rule of thumb, a concentration of 1%. What solvents do you use in thin layer chromatography? If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate, or methylene. What Is The Solvent In Tlc.
From www.chegg.com
Solved TLC plate A, below, represents the TLC of a compound What Is The Solvent In Tlc If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate, or methylene chloride. It is important that the solvent level is below the line with the spot on it. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate :. What Is The Solvent In Tlc.
From www.researchgate.net
TLC analysis results of extracts from different twophase solvent What Is The Solvent In Tlc The typical eluent for tlc is a mixture of an apolar solvent (typically. What solvents do you use in thin layer chromatography? If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate, or methylene chloride. When the spot of mixture is dry, the plate is stood. What Is The Solvent In Tlc.
From www.brainkart.com
Choice Of Solvent System in Thin Layer Chromatography (TLC) What Is The Solvent In Tlc A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : Ideally the vials will have a lid to minimize vapors and preserve the samples if tipped over (figure 2.23a). It is important that the solvent level is below the line with the spot on it. Using a capillary tube, a. As a rule. What Is The Solvent In Tlc.
From es.slideshare.net
Chromatography (paper chromatography and tlc) What Is The Solvent In Tlc If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate, or methylene chloride. The typical eluent for tlc is a mixture of an apolar solvent (typically. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic. What Is The Solvent In Tlc.
From www.researchgate.net
What are the good solvents for leaf extract’s TLC? ResearchGate What Is The Solvent In Tlc A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : The typical eluent for tlc is a mixture of an apolar solvent (typically. Using a capillary tube, a. It is important that the solvent level is below the line with the spot on it. In thin layer chromatography the solid phase (silica gel. What Is The Solvent In Tlc.
From www.researchgate.net
What are the good solvents for leaf extract’s TLC? ResearchGate What Is The Solvent In Tlc When the spot of mixture is dry, the plate is stood in a shallow layer of solvent in a covered beaker. Using a capillary tube, a. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : What solvents do you use in thin layer chromatography? If the sample is not already in solution,. What Is The Solvent In Tlc.
From chem.libretexts.org
2.3A Overview of TLC Chemistry LibreTexts What Is The Solvent In Tlc What solvents do you use in thin layer chromatography? The optimal concentration for tlc is typically determined empirically, but a good place to. Ideally the vials will have a lid to minimize vapors and preserve the samples if tipped over (figure 2.23a). When the spot of mixture is dry, the plate is stood in a shallow layer of solvent in. What Is The Solvent In Tlc.
From wirevutuinordinacy.z21.web.core.windows.net
Tlc Chromatography Diagram What Is The Solvent In Tlc In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. Ideally the vials will have a lid to minimize vapors and preserve the samples if tipped over (figure 2.23a). It is important that the solvent level is below the line with. What Is The Solvent In Tlc.
From www.researchgate.net
TLC plate run in solvent systems 'A' and 'C' 1 = Caloplaca What Is The Solvent In Tlc If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate, or methylene chloride. When the spot of mixture is dry, the plate is stood in a shallow layer of solvent in a covered beaker. As a rule of thumb, a concentration of 1%. The typical eluent. What Is The Solvent In Tlc.
From hxeemavye.blob.core.windows.net
Tlc Solvent Guide at David Richards blog What Is The Solvent In Tlc Using a capillary tube, a. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. As a rule of thumb, a concentration of 1%. When the spot of mixture is dry, the plate is stood in a shallow layer of solvent. What Is The Solvent In Tlc.
From www.reddit.com
TLC and solvent polarity!! What does it mean that it’s ‘too non polar What Is The Solvent In Tlc When the spot of mixture is dry, the plate is stood in a shallow layer of solvent in a covered beaker. The typical eluent for tlc is a mixture of an apolar solvent (typically. If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate, or methylene. What Is The Solvent In Tlc.
From www.chegg.com
Solved A sample is run on a TLC plate and gives a single What Is The Solvent In Tlc The optimal concentration for tlc is typically determined empirically, but a good place to. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate, or methylene chloride. What solvents. What Is The Solvent In Tlc.
From www.researchgate.net
Profile of extract in TLC (Solvent in nhexane/ethyl acetate 3/7 v/v What Is The Solvent In Tlc Using a capillary tube, a. It is important that the solvent level is below the line with the spot on it. When the spot of mixture is dry, the plate is stood in a shallow layer of solvent in a covered beaker. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : In. What Is The Solvent In Tlc.
From chemistryhall.com
Thin Layer Chromatography A Complete Guide to TLC What Is The Solvent In Tlc In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. Using a capillary tube, a. The optimal concentration for tlc is typically determined empirically, but a good place to. Ideally the vials will have a lid to minimize vapors and preserve. What Is The Solvent In Tlc.
From www.reddit.com
Reaction TLC analysis with acidic solvent What Is The Solvent In Tlc The optimal concentration for tlc is typically determined empirically, but a good place to. Ideally the vials will have a lid to minimize vapors and preserve the samples if tipped over (figure 2.23a). The typical eluent for tlc is a mixture of an apolar solvent (typically. A solvent that can be used for separating mixtures of strongly polar compounds is. What Is The Solvent In Tlc.
From microbenotes.com
Thin Layer Chromatography Principle, Parts, Steps, Uses What Is The Solvent In Tlc Ideally the vials will have a lid to minimize vapors and preserve the samples if tipped over (figure 2.23a). A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate,. What Is The Solvent In Tlc.
From www.scribd.com
Solvent TLC System PDF Thin Layer Chromatography Scientific What Is The Solvent In Tlc The optimal concentration for tlc is typically determined empirically, but a good place to. When the spot of mixture is dry, the plate is stood in a shallow layer of solvent in a covered beaker. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called. What Is The Solvent In Tlc.
From www.numerade.com
SOLVED Consider the TLC plate shown below Two substances, A and B What Is The Solvent In Tlc If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate, or methylene chloride. As a rule of thumb, a concentration of 1%. Ideally the vials will have a lid to minimize vapors and preserve the samples if tipped over (figure 2.23a). Using a capillary tube, a.. What Is The Solvent In Tlc.
From chemistryhall.com
Thin Layer Chromatography A Complete Guide to TLC What Is The Solvent In Tlc When the spot of mixture is dry, the plate is stood in a shallow layer of solvent in a covered beaker. What solvents do you use in thin layer chromatography? If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate, or methylene chloride. The typical eluent. What Is The Solvent In Tlc.
From www.researchgate.net
TLC tracing showing compounds in various solvent systems. TLC thin What Is The Solvent In Tlc Ideally the vials will have a lid to minimize vapors and preserve the samples if tipped over (figure 2.23a). Using a capillary tube, a. The typical eluent for tlc is a mixture of an apolar solvent (typically. When the spot of mixture is dry, the plate is stood in a shallow layer of solvent in a covered beaker. If the. What Is The Solvent In Tlc.
From www.researchgate.net
TLC of different fractions with standard quercetin; solvent system What Is The Solvent In Tlc As a rule of thumb, a concentration of 1%. The optimal concentration for tlc is typically determined empirically, but a good place to. What solvents do you use in thin layer chromatography? It is important that the solvent level is below the line with the spot on it. The typical eluent for tlc is a mixture of an apolar solvent. What Is The Solvent In Tlc.
From www.slideshare.net
Chromatography What Is The Solvent In Tlc The optimal concentration for tlc is typically determined empirically, but a good place to. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. The typical eluent for tlc is a mixture of an apolar solvent (typically. If the sample is. What Is The Solvent In Tlc.
From www.slideserve.com
PPT ThinLayer Chromatography PowerPoint Presentation ID649433 What Is The Solvent In Tlc The optimal concentration for tlc is typically determined empirically, but a good place to. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig.. What Is The Solvent In Tlc.
From owlcation.com
Thin Layer Chromatography (TLC) Principle and Procedure Owlcation What Is The Solvent In Tlc The typical eluent for tlc is a mixture of an apolar solvent (typically. When the spot of mixture is dry, the plate is stood in a shallow layer of solvent in a covered beaker. If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate, or methylene. What Is The Solvent In Tlc.
From chemistryhall.com
Thin Layer Chromatography A Complete Guide to TLC What Is The Solvent In Tlc It is important that the solvent level is below the line with the spot on it. If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate, or methylene chloride. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate :. What Is The Solvent In Tlc.
From mavink.com
Solvent Polarity Chart For Tlc What Is The Solvent In Tlc The typical eluent for tlc is a mixture of an apolar solvent (typically. When the spot of mixture is dry, the plate is stood in a shallow layer of solvent in a covered beaker. Ideally the vials will have a lid to minimize vapors and preserve the samples if tipped over (figure 2.23a). What solvents do you use in thin. What Is The Solvent In Tlc.
From www.makingmolecules.com
Thin Layer Chromatography (TLC) — Making Molecules What Is The Solvent In Tlc In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. As a rule of thumb, a concentration of 1%. It is important that the solvent level is below the line with the spot on it. A solvent that can be used. What Is The Solvent In Tlc.
From www.researchgate.net
TLC profile of T. involucrata L. Eluting solvents Fractions TLC solvent What Is The Solvent In Tlc It is important that the solvent level is below the line with the spot on it. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : The typical eluent for tlc is a mixture of an apolar solvent (typically. What solvents do you use in thin layer chromatography? In thin layer chromatography the. What Is The Solvent In Tlc.
From mavink.com
Solvent Polarity Chart For Tlc What Is The Solvent In Tlc As a rule of thumb, a concentration of 1%. Ideally the vials will have a lid to minimize vapors and preserve the samples if tipped over (figure 2.23a). What solvents do you use in thin layer chromatography? It is important that the solvent level is below the line with the spot on it. The typical eluent for tlc is a. What Is The Solvent In Tlc.
From www.slideshare.net
Tlc lecture What Is The Solvent In Tlc What solvents do you use in thin layer chromatography? If the sample is not already in solution, dissolve about 1 mg in 1 ml of a volatile solvent such as hexanes, ethyl acetate, or methylene chloride. Ideally the vials will have a lid to minimize vapors and preserve the samples if tipped over (figure 2.23a). When the spot of mixture. What Is The Solvent In Tlc.
From www.researchgate.net
Solvent mixtures assayed in TLC. Download Scientific Diagram What Is The Solvent In Tlc The optimal concentration for tlc is typically determined empirically, but a good place to. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : Using a capillary tube, a. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide,. What Is The Solvent In Tlc.
From www.researchgate.net
Different solvent system for TLC of plant extract Download Scientific What Is The Solvent In Tlc In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. As a rule of thumb, a concentration of 1%. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : What solvents do you use. What Is The Solvent In Tlc.