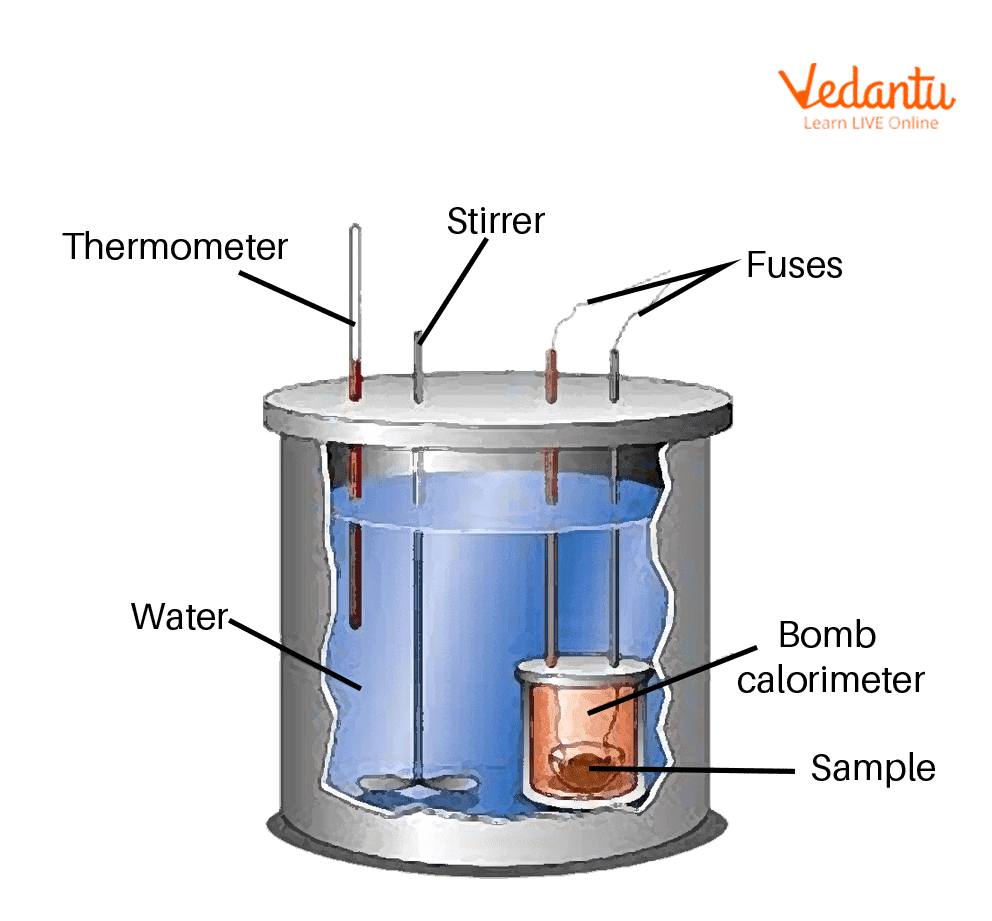
Bomb Calorimeter For Food . A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. what does a bomb calorimeter determine in a food sample? the foods are completely oxidized in the bomb calorimeter, to yield the end products: But how is this number determined? Carbon dioxide, water, oxides of nitrogen. The instrument used is called a bomb calorimeter. food labels contain the number of calories per serving. a bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat. the calories, or energy, from foods is measured using direct calorimetry. This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment.
from www.vedantu.com
This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment. the foods are completely oxidized in the bomb calorimeter, to yield the end products: But how is this number determined? the calories, or energy, from foods is measured using direct calorimetry. a bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Carbon dioxide, water, oxides of nitrogen. what does a bomb calorimeter determine in a food sample? The instrument used is called a bomb calorimeter. food labels contain the number of calories per serving.
Bomb Calorimeter Learn Important Terms and Concepts
Bomb Calorimeter For Food the calories, or energy, from foods is measured using direct calorimetry. the calories, or energy, from foods is measured using direct calorimetry. Carbon dioxide, water, oxides of nitrogen. The instrument used is called a bomb calorimeter. the foods are completely oxidized in the bomb calorimeter, to yield the end products: But how is this number determined? what does a bomb calorimeter determine in a food sample? The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. a bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat. food labels contain the number of calories per serving. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment.
From www.slideserve.com
PPT Chapter 6 Thermochemistry Energy Flow and Chemical Change Bomb Calorimeter For Food The instrument used is called a bomb calorimeter. Carbon dioxide, water, oxides of nitrogen. But how is this number determined? food labels contain the number of calories per serving. the foods are completely oxidized in the bomb calorimeter, to yield the end products: the calories, or energy, from foods is measured using direct calorimetry. A bomb calorimeter. Bomb Calorimeter For Food.
From dxobmbkmm.blob.core.windows.net
Bomb Calorimeter Used To Measure at Angela Johnson blog Bomb Calorimeter For Food A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. the foods are completely oxidized in the bomb calorimeter, to yield the end products: a bomb calorimeter is a device used to measure the heat of combustion of fuels,. Bomb Calorimeter For Food.
From www.bartleby.com
Answered A bomb calorimeter, or a constant… bartleby Bomb Calorimeter For Food The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment. The instrument used is called a bomb calorimeter. A bomb calorimeter is used to measure, under controlled conditions, the heat. Bomb Calorimeter For Food.
From dxobmbkmm.blob.core.windows.net
Bomb Calorimeter Used To Measure at Angela Johnson blog Bomb Calorimeter For Food what does a bomb calorimeter determine in a food sample? Carbon dioxide, water, oxides of nitrogen. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The instrument used is called a bomb calorimeter. the calories, or energy, from. Bomb Calorimeter For Food.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Bomb Calorimeter For Food the calories, or energy, from foods is measured using direct calorimetry. The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. But how is this number determined? the foods are completely oxidized in the bomb calorimeter, to yield the end products: a bomb calorimeter is a device. Bomb Calorimeter For Food.
From www.youtube.com
Estimation of Energy Available from Food(Bomb Calorimeter), Chemistry Bomb Calorimeter For Food A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment. The instrument used is called a bomb calorimeter. the foods. Bomb Calorimeter For Food.
From www.slideserve.com
PPT Measurement of Energy in Food and During Physical Activity Bomb Calorimeter For Food the calories, or energy, from foods is measured using direct calorimetry. the foods are completely oxidized in the bomb calorimeter, to yield the end products: The instrument used is called a bomb calorimeter. The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. A bomb calorimeter is used. Bomb Calorimeter For Food.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Bomb Calorimeter For Food But how is this number determined? This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment. The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. what does a bomb calorimeter determine in a food sample? the calories,. Bomb Calorimeter For Food.
From dir.indiamart.com
Bomb Calorimeters at Best Price in India Bomb Calorimeter For Food The instrument used is called a bomb calorimeter. Carbon dioxide, water, oxides of nitrogen. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. the foods are completely oxidized in the bomb calorimeter, to yield the end products: But how. Bomb Calorimeter For Food.
From foodtechnews.in
What Is Bomb Calorimeter🤔 Measurement of Energy Content in food Food Bomb Calorimeter For Food A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. But how is this number determined? The instrument used is called a bomb calorimeter. the calories, or energy, from foods is measured using direct calorimetry. the foods are completely. Bomb Calorimeter For Food.
From dxobmbkmm.blob.core.windows.net
Bomb Calorimeter Used To Measure at Angela Johnson blog Bomb Calorimeter For Food the calories, or energy, from foods is measured using direct calorimetry. The instrument used is called a bomb calorimeter. This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment. a bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of. Bomb Calorimeter For Food.
From ar.inspiredpencil.com
Bomb Calorimeter Bomb Calorimeter For Food The instrument used is called a bomb calorimeter. the foods are completely oxidized in the bomb calorimeter, to yield the end products: Carbon dioxide, water, oxides of nitrogen. what does a bomb calorimeter determine in a food sample? A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen. Bomb Calorimeter For Food.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation ID239015 Bomb Calorimeter For Food A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Carbon dioxide, water, oxides of nitrogen. The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. a bomb calorimeter is. Bomb Calorimeter For Food.
From www.slideserve.com
PPT Lesson 2 Role of Metabolism in Nutrition PowerPoint Bomb Calorimeter For Food The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. a bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat. what does a bomb calorimeter determine in a food sample? the calories, or energy, from. Bomb Calorimeter For Food.
From studylib.net
Bomb Calorimeter Lab Sheet Bomb Calorimeter For Food This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment. The instrument used is called a bomb calorimeter. The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. Carbon dioxide, water, oxides of nitrogen. A bomb calorimeter is used to. Bomb Calorimeter For Food.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness Bomb Calorimeter For Food food labels contain the number of calories per serving. what does a bomb calorimeter determine in a food sample? a bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat. But how is this number determined? the foods are completely oxidized in the bomb calorimeter,. Bomb Calorimeter For Food.
From www.studypool.com
SOLUTION Bomb calorimeter study material Studypool Bomb Calorimeter For Food The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. a bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat. the foods are completely oxidized in the bomb calorimeter, to yield the end products: what. Bomb Calorimeter For Food.
From dxobrspma.blob.core.windows.net
Calorimetry With Food at Roberto Whitney blog Bomb Calorimeter For Food Carbon dioxide, water, oxides of nitrogen. food labels contain the number of calories per serving. what does a bomb calorimeter determine in a food sample? The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. the calories, or energy, from foods is measured using direct calorimetry. . Bomb Calorimeter For Food.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimeter For Food This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment. what does a bomb calorimeter determine in a food sample? The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. The instrument used is called a bomb calorimeter. Carbon. Bomb Calorimeter For Food.
From www.indiamart.com
Isoperibol Bomb Calorimeter / For Food / Coal SDAC6000 / For Fuel Bomb Calorimeter For Food the calories, or energy, from foods is measured using direct calorimetry. what does a bomb calorimeter determine in a food sample? the foods are completely oxidized in the bomb calorimeter, to yield the end products: The instrument used is called a bomb calorimeter. The aim of this laboratory exercise is to apply the first law of thermodynamics. Bomb Calorimeter For Food.
From www.shutterstock.com
Bomb Calorimeter Vector Illustration Labeled Educational Stock Vector Bomb Calorimeter For Food Carbon dioxide, water, oxides of nitrogen. The instrument used is called a bomb calorimeter. food labels contain the number of calories per serving. the foods are completely oxidized in the bomb calorimeter, to yield the end products: The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. A. Bomb Calorimeter For Food.
From www.animalia-life.club
Food Calorimeter Bomb Calorimeter For Food the foods are completely oxidized in the bomb calorimeter, to yield the end products: This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment. what does a bomb calorimeter determine in a food sample? The instrument used is called a bomb calorimeter. food labels contain the number. Bomb Calorimeter For Food.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimeter For Food Carbon dioxide, water, oxides of nitrogen. This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment. the calories, or energy, from foods is measured using direct calorimetry. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in. Bomb Calorimeter For Food.
From dxobmbkmm.blob.core.windows.net
Bomb Calorimeter Used To Measure at Angela Johnson blog Bomb Calorimeter For Food This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment. the foods are completely oxidized in the bomb calorimeter, to yield the end products: the calories, or energy, from foods is measured using direct calorimetry. Carbon dioxide, water, oxides of nitrogen. The aim of this laboratory exercise is. Bomb Calorimeter For Food.
From nursekey.com
Energy Supply and Fitness Nurse Key Bomb Calorimeter For Food food labels contain the number of calories per serving. a bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded. Bomb Calorimeter For Food.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimeter For Food The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. what does a bomb calorimeter determine in a food sample? a bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat. The instrument used is called a. Bomb Calorimeter For Food.
From www.scienceabc.com
How Do We Calculate How Many Carbs Are In The Food? Bomb Calorimeter For Food what does a bomb calorimeter determine in a food sample? Carbon dioxide, water, oxides of nitrogen. This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment. The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. But how is. Bomb Calorimeter For Food.
From saylordotorg.github.io
Calorimetry Bomb Calorimeter For Food But how is this number determined? Carbon dioxide, water, oxides of nitrogen. The instrument used is called a bomb calorimeter. The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment.. Bomb Calorimeter For Food.
From www.slideserve.com
PPT Energy in food PowerPoint Presentation, free download ID5830775 Bomb Calorimeter For Food Carbon dioxide, water, oxides of nitrogen. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment. food labels contain the. Bomb Calorimeter For Food.
From chemlab.truman.edu
Parr 1341 Bomb Calorimeter Chem Lab Bomb Calorimeter For Food food labels contain the number of calories per serving. But how is this number determined? A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. This is a metal container inside which the food sample is burned in a sealed. Bomb Calorimeter For Food.
From www.youtube.com
Bomb Calorimeter Definition, Construction, Working & Uses YouTube Bomb Calorimeter For Food This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment. The instrument used is called a bomb calorimeter. The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. food labels contain the number of calories per serving. the. Bomb Calorimeter For Food.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter For Food This is a metal container inside which the food sample is burned in a sealed and pressurized pure oxygen environment. a bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat. The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the. Bomb Calorimeter For Food.
From www.thoughtco.com
Coffee Cup and Bomb Calorimetry Bomb Calorimeter For Food A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. But how is this number determined? Carbon dioxide, water, oxides of nitrogen. the foods are completely oxidized in the bomb calorimeter, to yield the end products: The aim of this. Bomb Calorimeter For Food.
From dxotpxhft.blob.core.windows.net
Food Calorimetry Lab High School at Angelina Britton blog Bomb Calorimeter For Food a bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat. The instrument used is called a bomb calorimeter. The aim of this laboratory exercise is to apply the first law of thermodynamics to determine the energy content (i.e. food labels contain the number of calories per. Bomb Calorimeter For Food.
From www.numerade.com
SOLVED A bomb calorimeter, or constant volume calorimeter; is a device Bomb Calorimeter For Food food labels contain the number of calories per serving. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. a bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions,. Bomb Calorimeter For Food.