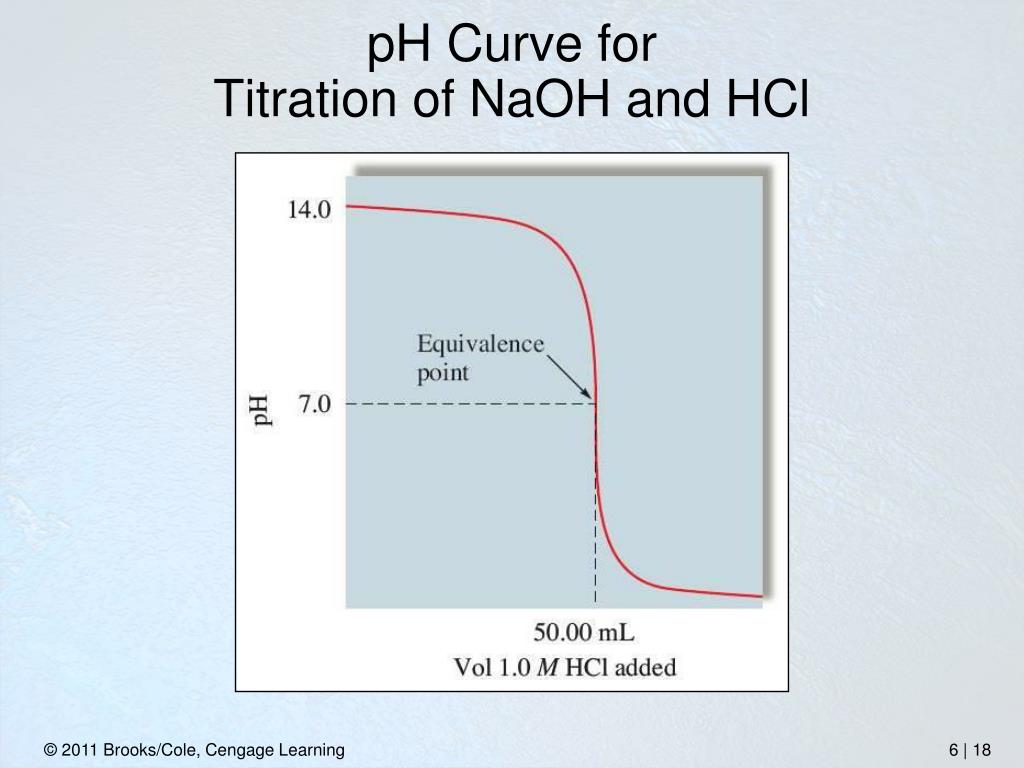
Titration Of Hcl And Naoh Equation . Hcl and naoh reacts in 1:1 ratio (in same amount). Updated on november 26, 2019. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: For instance, the combination of hydrochloric acid (hcl) and sodium hydroxide (naoh) results in an aqueous solution of sodium chloride (nacl), as represented by this equation: In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. The moles of acid will. To decide required amount (mol) and volume, the. [1,2] hcl + naoh → nacl + h 2 o \ce{naoh}\) is required to reach the end point when titrated. Important factors and equations of hcl + naoh reaction and its titration curve. Titrating sodium hydroxide with hydrochloric acid. Relating titrations to stoichiometric equations. Relating titrations to stoichiometry and limiting reagent problems. Suppose that a titration is performed and \(20.70 \:
from mungfali.com
[1,2] hcl + naoh → nacl + h 2 o Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: For instance, the combination of hydrochloric acid (hcl) and sodium hydroxide (naoh) results in an aqueous solution of sodium chloride (nacl), as represented by this equation: To decide required amount (mol) and volume, the. Hcl and naoh reacts in 1:1 ratio (in same amount). Important factors and equations of hcl + naoh reaction and its titration curve. Suppose that a titration is performed and \(20.70 \: Relating titrations to stoichiometry and limiting reagent problems. The moles of acid will. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in.
HCl NaOH Titration
Titration Of Hcl And Naoh Equation [1,2] hcl + naoh → nacl + h 2 o \ce{naoh}\) is required to reach the end point when titrated. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Relating titrations to stoichiometry and limiting reagent problems. Important factors and equations of hcl + naoh reaction and its titration curve. [1,2] hcl + naoh → nacl + h 2 o The moles of acid will. Suppose that a titration is performed and \(20.70 \: Titrating sodium hydroxide with hydrochloric acid. For instance, the combination of hydrochloric acid (hcl) and sodium hydroxide (naoh) results in an aqueous solution of sodium chloride (nacl), as represented by this equation: Relating titrations to stoichiometric equations. To decide required amount (mol) and volume, the. Hcl and naoh reacts in 1:1 ratio (in same amount). Updated on november 26, 2019. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as:
From www.slideshare.net
5 3 and 5 4 Titration Of Hcl And Naoh Equation To decide required amount (mol) and volume, the. Relating titrations to stoichiometric equations. Updated on november 26, 2019. Hcl and naoh reacts in 1:1 ratio (in same amount). Titrating sodium hydroxide with hydrochloric acid. [1,2] hcl + naoh → nacl + h 2 o The moles of acid will. \ce{naoh}\) is required to reach the end point when titrated. For. Titration Of Hcl And Naoh Equation.
From mungfali.com
HCl NaOH Titration Titration Of Hcl And Naoh Equation Hcl and naoh reacts in 1:1 ratio (in same amount). The moles of acid will. \ce{naoh}\) is required to reach the end point when titrated. Important factors and equations of hcl + naoh reaction and its titration curve. For instance, the combination of hydrochloric acid (hcl) and sodium hydroxide (naoh) results in an aqueous solution of sodium chloride (nacl), as. Titration Of Hcl And Naoh Equation.
From www.youtube.com
Titration calculation 1 NaOH Vs HCl YouTube Titration Of Hcl And Naoh Equation In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. [1,2] hcl + naoh → nacl + h 2 o Relating titrations to stoichiometric equations. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Important factors and equations of hcl. Titration Of Hcl And Naoh Equation.
From www.thinkswap.com
Titration of Sodium Hydroxide with Hydrochloric acid FSC107 General Titration Of Hcl And Naoh Equation Suppose that a titration is performed and \(20.70 \: Updated on november 26, 2019. To decide required amount (mol) and volume, the. Hcl and naoh reacts in 1:1 ratio (in same amount). [1,2] hcl + naoh → nacl + h 2 o Titrating sodium hydroxide with hydrochloric acid. \ce{naoh}\) is required to reach the end point when titrated. Since hcl. Titration Of Hcl And Naoh Equation.
From www.chegg.com
Solved A) The Reaction Of HCl With NaOH Is Represented By... Titration Of Hcl And Naoh Equation [1,2] hcl + naoh → nacl + h 2 o \ce{naoh}\) is required to reach the end point when titrated. Important factors and equations of hcl + naoh reaction and its titration curve. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Hcl and naoh reacts in 1:1 ratio (in same. Titration Of Hcl And Naoh Equation.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download Titration Of Hcl And Naoh Equation In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Suppose that a titration is performed and \(20.70 \: Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: For instance, the combination of hydrochloric acid (hcl) and sodium hydroxide (naoh). Titration Of Hcl And Naoh Equation.
From byjus.com
The graph of pH during the titration of NaOH and HCl Titration Of Hcl And Naoh Equation Relating titrations to stoichiometry and limiting reagent problems. The moles of acid will. [1,2] hcl + naoh → nacl + h 2 o Important factors and equations of hcl + naoh reaction and its titration curve. Titrating sodium hydroxide with hydrochloric acid. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite. Titration Of Hcl And Naoh Equation.
From www.chegg.com
Solved Titration of HCl with standardized NaOH Titration Of Hcl And Naoh Equation To decide required amount (mol) and volume, the. Important factors and equations of hcl + naoh reaction and its titration curve. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Relating titrations to stoichiometry and limiting reagent problems. For instance, the combination of hydrochloric acid (hcl) and sodium. Titration Of Hcl And Naoh Equation.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Titration Of Hcl And Naoh Equation Titrating sodium hydroxide with hydrochloric acid. The moles of acid will. Relating titrations to stoichiometric equations. \ce{naoh}\) is required to reach the end point when titrated. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. To decide required amount (mol) and volume, the. Relating titrations to stoichiometry and limiting reagent problems.. Titration Of Hcl And Naoh Equation.
From mungfali.com
HCl And NaOH Equation Titration Of Hcl And Naoh Equation Updated on november 26, 2019. [1,2] hcl + naoh → nacl + h 2 o Important factors and equations of hcl + naoh reaction and its titration curve. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. To decide required amount (mol) and volume, the. For instance, the combination of hydrochloric. Titration Of Hcl And Naoh Equation.
From loehmowzt.blob.core.windows.net
Indicator Used In Titration Of Naoh And Hcl at Dan Carlson blog Titration Of Hcl And Naoh Equation Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: To decide required amount (mol) and volume, the. [1,2] hcl + naoh → nacl + h 2 o For instance, the combination of hydrochloric acid (hcl) and sodium hydroxide (naoh) results in an aqueous solution of sodium chloride (nacl),. Titration Of Hcl And Naoh Equation.
From exyptikle.blob.core.windows.net
Chemical Reaction Between Hydrochloric Acid And Sodium Hypochlorite at Titration Of Hcl And Naoh Equation Important factors and equations of hcl + naoh reaction and its titration curve. The moles of acid will. Titrating sodium hydroxide with hydrochloric acid. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Relating titrations to stoichiometry and limiting reagent problems. Since hcl and naoh fully dissociate into their ion components,. Titration Of Hcl And Naoh Equation.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Titration Of Hcl And Naoh Equation Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: The moles of acid will. Relating titrations to stoichiometry and limiting reagent problems. \ce{naoh}\) is required to reach the end point when titrated. [1,2] hcl + naoh → nacl + h 2 o Updated on november 26, 2019. Hcl. Titration Of Hcl And Naoh Equation.
From mungfali.com
HCl NaOH Titration Titration Of Hcl And Naoh Equation Hcl and naoh reacts in 1:1 ratio (in same amount). Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Suppose that a titration is performed and \(20.70 \: The moles of acid will. Relating titrations to stoichiometric equations. For instance, the combination of hydrochloric acid (hcl) and sodium. Titration Of Hcl And Naoh Equation.
From www.youtube.com
Lab 2 (Titration of HCl with Na2CO3) YouTube Titration Of Hcl And Naoh Equation Relating titrations to stoichiometry and limiting reagent problems. \ce{naoh}\) is required to reach the end point when titrated. Updated on november 26, 2019. Hcl and naoh reacts in 1:1 ratio (in same amount). In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. To decide required amount (mol) and volume, the. Titrating. Titration Of Hcl And Naoh Equation.
From studylib.net
Titration of HCl with NaOH Titration Of Hcl And Naoh Equation Relating titrations to stoichiometry and limiting reagent problems. Suppose that a titration is performed and \(20.70 \: [1,2] hcl + naoh → nacl + h 2 o Relating titrations to stoichiometric equations. To decide required amount (mol) and volume, the. Titrating sodium hydroxide with hydrochloric acid. For instance, the combination of hydrochloric acid (hcl) and sodium hydroxide (naoh) results in. Titration Of Hcl And Naoh Equation.
From mungfali.com
HCl NaOH Titration Titration Of Hcl And Naoh Equation Important factors and equations of hcl + naoh reaction and its titration curve. Suppose that a titration is performed and \(20.70 \: Titrating sodium hydroxide with hydrochloric acid. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: To decide required amount (mol) and volume, the. For instance, the. Titration Of Hcl And Naoh Equation.
From ceplknbb.blob.core.windows.net
Acid Base Titration Questions at Deborah Wedel blog Titration Of Hcl And Naoh Equation Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: For instance, the combination of hydrochloric acid (hcl) and sodium hydroxide (naoh) results in an aqueous solution of sodium chloride (nacl), as represented by this equation: Suppose that a titration is performed and \(20.70 \: Updated on november 26,. Titration Of Hcl And Naoh Equation.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Titration Of Hcl And Naoh Equation Important factors and equations of hcl + naoh reaction and its titration curve. Titrating sodium hydroxide with hydrochloric acid. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Updated on november 26, 2019. Relating titrations to stoichiometric equations. To decide required amount (mol) and volume, the. \ce{naoh}\) is required to reach. Titration Of Hcl And Naoh Equation.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Titration Of Hcl And Naoh Equation To decide required amount (mol) and volume, the. [1,2] hcl + naoh → nacl + h 2 o For instance, the combination of hydrochloric acid (hcl) and sodium hydroxide (naoh) results in an aqueous solution of sodium chloride (nacl), as represented by this equation: Titrating sodium hydroxide with hydrochloric acid. Updated on november 26, 2019. Hcl and naoh reacts in. Titration Of Hcl And Naoh Equation.
From byjus.com
24.Why their is difference when naoh and na2co3 are titrated with Titration Of Hcl And Naoh Equation Relating titrations to stoichiometry and limiting reagent problems. Titrating sodium hydroxide with hydrochloric acid. For instance, the combination of hydrochloric acid (hcl) and sodium hydroxide (naoh) results in an aqueous solution of sodium chloride (nacl), as represented by this equation: In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Relating titrations. Titration Of Hcl And Naoh Equation.
From www.visionlearning.com
Ácidos y Bases I Chemistry Visionlearning Titration Of Hcl And Naoh Equation The moles of acid will. Important factors and equations of hcl + naoh reaction and its titration curve. \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \: To decide required amount (mol) and volume, the. [1,2] hcl + naoh → nacl + h 2 o Updated on november 26, 2019.. Titration Of Hcl And Naoh Equation.
From www.numerade.com
SOLVED 4) (4 pts) In lab you titrated HCl with NaOH Sketch the Titration Of Hcl And Naoh Equation For instance, the combination of hydrochloric acid (hcl) and sodium hydroxide (naoh) results in an aqueous solution of sodium chloride (nacl), as represented by this equation: \ce{naoh}\) is required to reach the end point when titrated. Relating titrations to stoichiometry and limiting reagent problems. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we. Titration Of Hcl And Naoh Equation.
From meetingtarget11.gitlab.io
Ideal Naoh Hcl Balanced Equation What Is The Chemical For Photosynthesis Titration Of Hcl And Naoh Equation Updated on november 26, 2019. For instance, the combination of hydrochloric acid (hcl) and sodium hydroxide (naoh) results in an aqueous solution of sodium chloride (nacl), as represented by this equation: In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Hcl and naoh reacts in 1:1 ratio (in same amount). The. Titration Of Hcl And Naoh Equation.
From www.youtube.com
Titration of HCl with NaOH YouTube Titration Of Hcl And Naoh Equation Important factors and equations of hcl + naoh reaction and its titration curve. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. \ce{naoh}\) is required to reach the end point when titrated. Updated on november 26, 2019. Relating titrations to stoichiometry and limiting reagent problems. Suppose that a titration is performed. Titration Of Hcl And Naoh Equation.
From klaimjxhd.blob.core.windows.net
Titration Between Hcl And Naoh at Tamika Spear blog Titration Of Hcl And Naoh Equation The moles of acid will. Suppose that a titration is performed and \(20.70 \: Relating titrations to stoichiometry and limiting reagent problems. To decide required amount (mol) and volume, the. Hcl and naoh reacts in 1:1 ratio (in same amount). In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Important factors. Titration Of Hcl And Naoh Equation.
From mungfali.com
HCl NaOH Titration Titration Of Hcl And Naoh Equation Hcl and naoh reacts in 1:1 ratio (in same amount). Relating titrations to stoichiometric equations. For instance, the combination of hydrochloric acid (hcl) and sodium hydroxide (naoh) results in an aqueous solution of sodium chloride (nacl), as represented by this equation: \ce{naoh}\) is required to reach the end point when titrated. To decide required amount (mol) and volume, the. Since. Titration Of Hcl And Naoh Equation.
From www.numerade.com
SOLVED Write the chemical equation for the titration of HCl and NaOH Titration Of Hcl And Naoh Equation [1,2] hcl + naoh → nacl + h 2 o Relating titrations to stoichiometric equations. Hcl and naoh reacts in 1:1 ratio (in same amount). To decide required amount (mol) and volume, the. Suppose that a titration is performed and \(20.70 \: Important factors and equations of hcl + naoh reaction and its titration curve. Updated on november 26, 2019.. Titration Of Hcl And Naoh Equation.
From exyptikle.blob.core.windows.net
Chemical Reaction Between Hydrochloric Acid And Sodium Hypochlorite at Titration Of Hcl And Naoh Equation Relating titrations to stoichiometric equations. To decide required amount (mol) and volume, the. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Important factors and equations of hcl + naoh reaction and its titration curve. \ce{naoh}\) is required to reach the end point when titrated. Titrating sodium hydroxide with hydrochloric acid.. Titration Of Hcl And Naoh Equation.
From mungfali.com
Titration Curve HCl And NaOH Titration Of Hcl And Naoh Equation Relating titrations to stoichiometry and limiting reagent problems. Updated on november 26, 2019. Suppose that a titration is performed and \(20.70 \: Titrating sodium hydroxide with hydrochloric acid. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. \ce{naoh}\) is required to reach the end point when titrated. Important factors and equations. Titration Of Hcl And Naoh Equation.
From www.chemistryscl.com
NaOH and HCl Titration Curves Selecting Indicators Titration Of Hcl And Naoh Equation Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \: Important factors and equations of hcl + naoh reaction and its titration curve. Relating titrations to stoichiometric equations. Updated on. Titration Of Hcl And Naoh Equation.
From www.numerade.com
SOLVED 2) Write the chemical equation for the titration of HCI with Titration Of Hcl And Naoh Equation Titrating sodium hydroxide with hydrochloric acid. \ce{naoh}\) is required to reach the end point when titrated. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Suppose that a titration is performed and \(20.70 \: The moles of acid will. Updated on november 26, 2019. Since hcl and naoh fully dissociate into. Titration Of Hcl And Naoh Equation.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration Of Hcl And Naoh Equation Updated on november 26, 2019. Suppose that a titration is performed and \(20.70 \: In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. To decide required amount (mol) and volume, the. The moles of acid will. Hcl and naoh reacts in 1:1 ratio (in same amount). For instance, the combination of. Titration Of Hcl And Naoh Equation.
From loehmowzt.blob.core.windows.net
Indicator Used In Titration Of Naoh And Hcl at Dan Carlson blog Titration Of Hcl And Naoh Equation [1,2] hcl + naoh → nacl + h 2 o Relating titrations to stoichiometric equations. Updated on november 26, 2019. To decide required amount (mol) and volume, the. Suppose that a titration is performed and \(20.70 \: In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Important factors and equations of. Titration Of Hcl And Naoh Equation.
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Titration Of Hcl And Naoh Equation Suppose that a titration is performed and \(20.70 \: Relating titrations to stoichiometry and limiting reagent problems. For instance, the combination of hydrochloric acid (hcl) and sodium hydroxide (naoh) results in an aqueous solution of sodium chloride (nacl), as represented by this equation: The moles of acid will. Titrating sodium hydroxide with hydrochloric acid. \ce{naoh}\) is required to reach the. Titration Of Hcl And Naoh Equation.