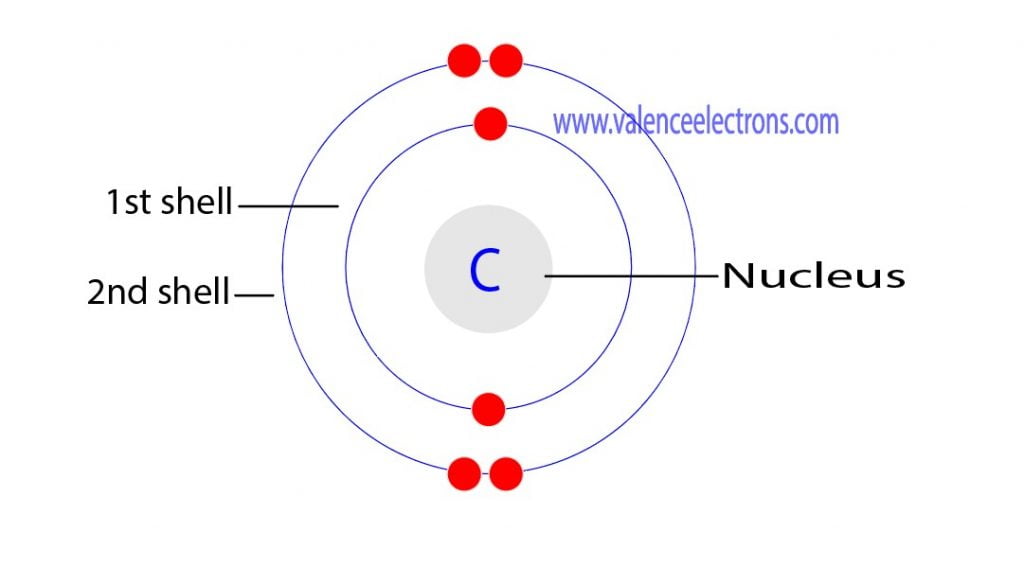
What Is Carbon Electron Shell Configuration . The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. By hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Following hydrogen is the noble gas helium, which has an atomic number of 2. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons). Electron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. Commonly, the electron configuration is used to describe. For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k shell and 4 in the next l shell. Here is the electron configuration for helium: The electron configuration and the orbital diagram are:
from valenceelectrons.com
The electron configuration and the orbital diagram are: Commonly, the electron configuration is used to describe. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k shell and 4 in the next l shell. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Following hydrogen is the noble gas helium, which has an atomic number of 2. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. Here is the electron configuration for helium: Electron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. By hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c.
How to Write the Electron Configuration for Carbon (C)?
What Is Carbon Electron Shell Configuration By hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Here is the electron configuration for helium: Commonly, the electron configuration is used to describe. The electron configuration and the orbital diagram are: By hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k shell and 4 in the next l shell. Following hydrogen is the noble gas helium, which has an atomic number of 2. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons). The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Electron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose.
From valenceelectrons.com
How to Write the Electron Configuration for Carbon (C)? What Is Carbon Electron Shell Configuration The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons). The electron configuration and the orbital diagram are: By hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. This electron configuration calculator will. What Is Carbon Electron Shell Configuration.
From enginerileyoutsport.z14.web.core.windows.net
Complete Electron Configuration For Carbon What Is Carbon Electron Shell Configuration The electron configuration and the orbital diagram are: Electron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. The electron configurations of silicon (14. What Is Carbon Electron Shell Configuration.
From diagramdataseminated.z21.web.core.windows.net
Electronic Configuration Of Carbon What Is Carbon Electron Shell Configuration By hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Commonly, the electron configuration is used to describe. Following hydrogen is the noble gas helium, which has an atomic number of 2. Here is the electron configuration for helium: For example, the electron. What Is Carbon Electron Shell Configuration.
From www.benjamin-mills.com
Electron configurations What Is Carbon Electron Shell Configuration For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k shell and 4 in the next l shell. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe. Here is. What Is Carbon Electron Shell Configuration.
From www.dreamstime.com
Carbon Atom Molecular Structure Labels Stock Vector Illustration of What Is Carbon Electron Shell Configuration Commonly, the electron configuration is used to describe. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. Here is the. What Is Carbon Electron Shell Configuration.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts What Is Carbon Electron Shell Configuration For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k shell and 4 in the next l shell. Here is the electron configuration for helium: Electron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. By hund’s rule, the electron configuration. What Is Carbon Electron Shell Configuration.
From mavink.com
Carbon Electron Shell Diagram What Is Carbon Electron Shell Configuration The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons). Electron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k shell. What Is Carbon Electron Shell Configuration.
From www.dreamstime.com
Carbon Element 6 Electron Configuration Vector Illustration Diagram What Is Carbon Electron Shell Configuration For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k shell and 4 in the next l shell. Here is the electron configuration for helium: Commonly, the electron configuration is used to describe. The electron configuration of an atom is the representation of the arrangement of electrons distributed among. What Is Carbon Electron Shell Configuration.
From www.alamy.com
Carbon atoms hires stock photography and images Alamy What Is Carbon Electron Shell Configuration For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k shell and 4 in the next l shell. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. By hund’s rule,. What Is Carbon Electron Shell Configuration.
From www.slideserve.com
PPT Orbital Filling Electron Configurations PowerPoint Presentation What Is Carbon Electron Shell Configuration The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Here is the electron configuration for helium: For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k shell and 4 in the next l shell. The electron configurations. What Is Carbon Electron Shell Configuration.
From mychem.co.uk
The periodic table 2 Mychem What Is Carbon Electron Shell Configuration Here is the electron configuration for helium: By hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Commonly, the electron configuration is used to describe. The electron configuration and the orbital diagram are: For example, the electron configuration of carbon atom is written. What Is Carbon Electron Shell Configuration.
From www.britannica.com
Electron shell Definition & Facts Britannica What Is Carbon Electron Shell Configuration Electron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons). For. What Is Carbon Electron Shell Configuration.
From www.newtondesk.com
Carbon Element With Reaction, Properties, Uses, & Price Periodic Table What Is Carbon Electron Shell Configuration Electron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons). Following hydrogen is the noble gas helium, which has an atomic number of 2. This electron configuration calculator will instantly show you. What Is Carbon Electron Shell Configuration.
From www.shutterstock.com
Vector Illustration Carbon Electron Shell Configuration Stock Vector What Is Carbon Electron Shell Configuration Commonly, the electron configuration is used to describe. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons). Following hydrogen is the noble gas helium, which has an atomic number of 2. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. What Is Carbon Electron Shell Configuration.
From ar.inspiredpencil.com
Carbon Electron Configuration What Is Carbon Electron Shell Configuration The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons). For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k shell and 4 in the next l shell. By hund’s rule, the electron configuration of carbon, which is 1s. What Is Carbon Electron Shell Configuration.
From www.goodscience.com.au
Electron Configuration (Elements 120) Good Science What Is Carbon Electron Shell Configuration By hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Electron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17. What Is Carbon Electron Shell Configuration.
From pixels.com
Carbon Electron Configuration Photograph by Photo What Is Carbon Electron Shell Configuration Following hydrogen is the noble gas helium, which has an atomic number of 2. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Electron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. Here is the electron configuration for helium:. What Is Carbon Electron Shell Configuration.
From www.vectorstock.com
Diagram representation of the element carbon Vector Image What Is Carbon Electron Shell Configuration Electron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. Here is the electron configuration for helium: For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k shell and 4 in the next l shell. Following hydrogen is the noble gas. What Is Carbon Electron Shell Configuration.
From sciencenotes.org
List of Electron Configurations of Elements What Is Carbon Electron Shell Configuration The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons). The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. Following hydrogen is the noble gas helium, which has an atomic number. What Is Carbon Electron Shell Configuration.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell What Is Carbon Electron Shell Configuration For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k shell and 4 in the next l shell. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. By hund’s rule, the electron configuration of carbon, which is. What Is Carbon Electron Shell Configuration.
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements What Is Carbon Electron Shell Configuration Following hydrogen is the noble gas helium, which has an atomic number of 2. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons). The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. This electron configuration calculator will. What Is Carbon Electron Shell Configuration.
From valenceelectrons.com
How to Write the Electron Configuration for Carbon (C)? What Is Carbon Electron Shell Configuration By hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Following hydrogen is the noble gas helium, which has an atomic number. What Is Carbon Electron Shell Configuration.
From www.youtube.com
Carbon electronic configuration How to Write Carbon electronic What Is Carbon Electron Shell Configuration Electron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. Here is the electron configuration for helium: By hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. The electron configurations of silicon (14 electrons), phosphorus. What Is Carbon Electron Shell Configuration.
From www.slideserve.com
PPT Organic Molecules The Building Blocks of Life PowerPoint What Is Carbon Electron Shell Configuration Following hydrogen is the noble gas helium, which has an atomic number of 2. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons). The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Electron configurations have a standard. What Is Carbon Electron Shell Configuration.
From iperiodictable.com
Orbital Diagram For Carbon (C) Carbon Electron Configuration What Is Carbon Electron Shell Configuration The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k shell and 4 in the next l shell. Following hydrogen is. What Is Carbon Electron Shell Configuration.
From www.istockphoto.com
Carbon Electron Shell Stock Illustration Download Image Now What Is Carbon Electron Shell Configuration By hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. The electron configuration and the. What Is Carbon Electron Shell Configuration.
From www.webelements.com
Elements Periodic Table » Carbon » properties of free atoms What Is Carbon Electron Shell Configuration For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k shell and 4 in the next l shell. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Commonly, the electron configuration is used to describe. The electron. What Is Carbon Electron Shell Configuration.
From www.pinterest.com.mx
Electron configuration, Carbon element, How to memorize things What Is Carbon Electron Shell Configuration Commonly, the electron configuration is used to describe. For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k shell and 4 in the next l shell. The electron configuration and the orbital diagram are: The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17. What Is Carbon Electron Shell Configuration.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements What Is Carbon Electron Shell Configuration The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons). Commonly, the electron configuration is used to describe. Electron configurations have a. What Is Carbon Electron Shell Configuration.
From chemistry291.blogspot.com
What Is the Carbon(C) Electron Configuration? What Is Carbon Electron Shell Configuration The electron configuration and the orbital diagram are: Commonly, the electron configuration is used to describe. Here is the electron configuration for helium: The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. For example, the electron configuration of carbon atom is. What Is Carbon Electron Shell Configuration.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer What Is Carbon Electron Shell Configuration The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. By hund’s rule, the electron configuration of carbon, which is 1s. What Is Carbon Electron Shell Configuration.
From general.chemistrysteps.com
Pauli Exclusion Principle Chemistry Steps What Is Carbon Electron Shell Configuration The electron configuration and the orbital diagram are: Electron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Following hydrogen is the noble gas helium, which has an atomic number of. What Is Carbon Electron Shell Configuration.
From www.chemistryland.com
Electron Configuration What Is Carbon Electron Shell Configuration The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. Electron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. What Is Carbon Electron Shell Configuration.
From mavink.com
Orbital Diagram Of Carbon What Is Carbon Electron Shell Configuration Here is the electron configuration for helium: This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. The electron configuration and the orbital diagram are: The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Electron configurations have. What Is Carbon Electron Shell Configuration.
From mavink.com
Carbon Electron Shell Diagram What Is Carbon Electron Shell Configuration The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. The electron configuration and the orbital diagram are: The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons). Commonly, the electron configuration. What Is Carbon Electron Shell Configuration.