Increasing The Concentration Of Ions In An Electrolyte Solution . There is direct relation between ion concentration and the current density limit, caused by ion diffusion. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. The larger the concentration of ions, the better the solutions. In the limiting state, all.
from www.youtube.com
Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : There is direct relation between ion concentration and the current density limit, caused by ion diffusion. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). In the limiting state, all. The larger the concentration of ions, the better the solutions. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases.
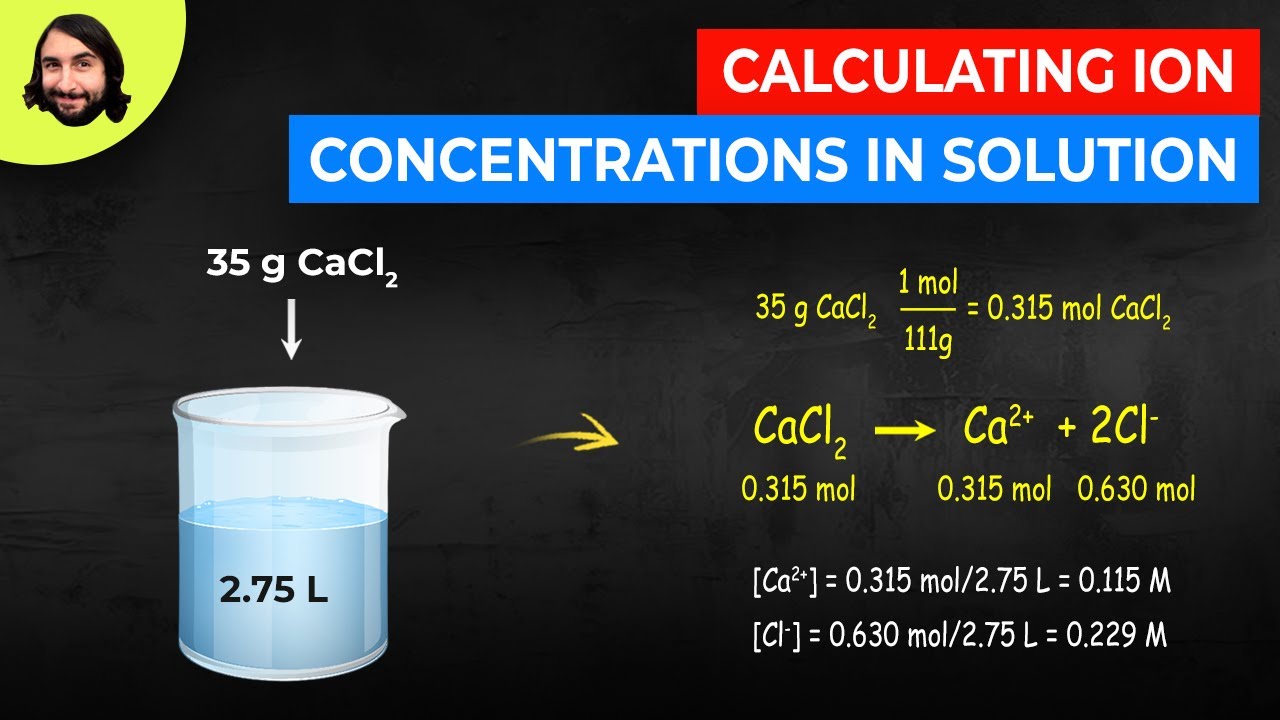
Calculating Ion Concentrations in Solution YouTube
Increasing The Concentration Of Ions In An Electrolyte Solution There is direct relation between ion concentration and the current density limit, caused by ion diffusion. In the limiting state, all. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. There is direct relation between ion concentration and the current density limit, caused by ion diffusion. The larger the concentration of ions, the better the solutions. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases.
From www.slideserve.com
PPT SOLUTIONS OF ELECTROLYTES PowerPoint Presentation, free download Increasing The Concentration Of Ions In An Electrolyte Solution Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. There is direct. Increasing The Concentration Of Ions In An Electrolyte Solution.
From slideplayer.com
Types of Solutions. ppt download Increasing The Concentration Of Ions In An Electrolyte Solution In the limiting state, all. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. The larger the concentration of ions, the better the solutions. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the.. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.youtube.com
4.1 Solutions, Aqueous Solutions, Electrolytes & Concentrations YouTube Increasing The Concentration Of Ions In An Electrolyte Solution In the limiting state, all. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : There is direct relation between ion concentration and the current density limit, caused by ion diffusion. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. The larger the concentration of ions, the better the solutions. When ionic compounds dissolve in. Increasing The Concentration Of Ions In An Electrolyte Solution.
From courses.lumenlearning.com
Electrolysis Chemistry Increasing The Concentration Of Ions In An Electrolyte Solution Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.youtube.com
Ion Concentration in Solutions From Molarity, Chemistry Practice Increasing The Concentration Of Ions In An Electrolyte Solution Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. In the limiting state, all. There is direct relation between ion concentration and the current density limit, caused by ion diffusion. Solutions may also conduct electricity. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.youtube.com
Calculating Ion Concentrations in Solution YouTube Increasing The Concentration Of Ions In An Electrolyte Solution The larger the concentration of ions, the better the solutions. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. An electrolyte solution conducts electricity because of the movement. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.slideshare.net
Chapter 6 electrochemistry Increasing The Concentration Of Ions In An Electrolyte Solution The larger the concentration of ions, the better the solutions. There is direct relation between ion concentration and the current density limit, caused by ion diffusion. In the limiting state, all. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. An electrolyte solution conducts electricity because of the movement of ions in. Increasing The Concentration Of Ions In An Electrolyte Solution.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Increasing The Concentration Of Ions In An Electrolyte Solution An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. In the limiting state, all. There is direct relation between. Increasing The Concentration Of Ions In An Electrolyte Solution.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes Increasing The Concentration Of Ions In An Electrolyte Solution Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Solutions may also conduct electricity if they contain. Increasing The Concentration Of Ions In An Electrolyte Solution.
From pediaa.com
Difference Between Strong and Weak Electrolytes Definition Increasing The Concentration Of Ions In An Electrolyte Solution The larger the concentration of ions, the better the solutions. In the limiting state, all. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. There is direct relation between ion concentration and the current density limit, caused by ion diffusion. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : Solutions may also conduct electricity. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.numerade.com
SOLVEDCalculate the concentration of all ions present in each of the Increasing The Concentration Of Ions In An Electrolyte Solution Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. An electrolyte solution conducts electricity because of the movement of ions in the solution (see. Increasing The Concentration Of Ions In An Electrolyte Solution.
From slideplayer.com
Concentrations of Solutions ppt download Increasing The Concentration Of Ions In An Electrolyte Solution An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). There is direct relation between ion concentration and the current density limit, caused by ion diffusion. The larger the concentration of ions, the better the solutions. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Property. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.researchgate.net
Ion concentration profile for a 11 electrolyte solution with c b Increasing The Concentration Of Ions In An Electrolyte Solution An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The larger the concentration of ions, the better the solutions. In the limiting state, all. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Solutions may also conduct electricity if they contain dissolved ions, with conductivity. Increasing The Concentration Of Ions In An Electrolyte Solution.
From atlas-scientific.com
How Do Ions Increase Conductivity? Atlas Scientific Increasing The Concentration Of Ions In An Electrolyte Solution Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. There is direct relation between ion concentration and. Increasing The Concentration Of Ions In An Electrolyte Solution.
From chemistryedu.org
Electrochemistry Variation of Conductivity and Molar Conductivity Increasing The Concentration Of Ions In An Electrolyte Solution Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. In the limiting state, all. There is direct relation between. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.researchgate.net
(a) Change in concentration of Li ions in the electrolyte and the Increasing The Concentration Of Ions In An Electrolyte Solution Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. The larger the concentration of ions, the better the solutions. In the limiting state, all. When ionic compounds dissolve in water, the ions in the solid. Increasing The Concentration Of Ions In An Electrolyte Solution.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Increasing The Concentration Of Ions In An Electrolyte Solution The larger the concentration of ions, the better the solutions. There is direct relation between ion concentration and the current density limit, caused by ion diffusion. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Solutions may also conduct electricity if they contain dissolved ions, with. Increasing The Concentration Of Ions In An Electrolyte Solution.
From apchemistryatgwhs.yolasite.com
APChemistryatGWHS Increasing The Concentration Of Ions In An Electrolyte Solution Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : The larger the concentration of ions, the better the solutions. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. There is direct relation between ion concentration and the. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.numerade.com
SOLVEDA liter of 0.1 M solution of NaOH, which is a strong electrolyte Increasing The Concentration Of Ions In An Electrolyte Solution Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. There is direct relation between ion concentration and the current density limit, caused by ion diffusion. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. When ionic compounds dissolve in water, the ions in the. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.youtube.com
Lab 9 Solutions, Electrolytes and Concentrations YouTube Increasing The Concentration Of Ions In An Electrolyte Solution When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. There is direct relation between ion concentration and the current density limit, caused by ion diffusion. The larger the concentration of ions, the better the solutions. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.researchgate.net
Effect of Li ion concentration of in the electrolyte. (a) Rate of Increasing The Concentration Of Ions In An Electrolyte Solution Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). There is direct. Increasing The Concentration Of Ions In An Electrolyte Solution.
From slideplayer.com
Electrolytes, Weak and Strong ppt download Increasing The Concentration Of Ions In An Electrolyte Solution In the limiting state, all. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.researchgate.net
Concentration of manganese ions in the electrolyte solution vs the Increasing The Concentration Of Ions In An Electrolyte Solution In the limiting state, all. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. There is direct relation between ion concentration and the current. Increasing The Concentration Of Ions In An Electrolyte Solution.
From flatworldknowledge.lardbucket.org
Aqueous Solutions Increasing The Concentration Of Ions In An Electrolyte Solution Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). There is direct relation between ion concentration and the current density limit, caused by ion diffusion. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : In the limiting state,. Increasing The Concentration Of Ions In An Electrolyte Solution.
From users.highland.edu
Solution Concentrations Increasing The Concentration Of Ions In An Electrolyte Solution In the limiting state, all. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. The larger the concentration of ions, the better the solutions. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : When ionic compounds dissolve. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Increasing The Concentration Of Ions In An Electrolyte Solution When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. In the limiting state, all. The larger the concentration of ions, the better the solutions.. Increasing The Concentration Of Ions In An Electrolyte Solution.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Increasing The Concentration Of Ions In An Electrolyte Solution Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : The larger the concentration of ions, the better the solutions. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. An electrolyte solution conducts electricity because of the movement. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.slideserve.com
PPT Ch4 Types of Chemical Reaction and Solutions Ch4.1 4.3 Water Increasing The Concentration Of Ions In An Electrolyte Solution Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. The larger the concentration of ions, the better the solutions. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. There is direct relation between ion concentration and the. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.researchgate.net
(a) Electrochemical stability window of LiTFSIH2O electrolytes. (b Increasing The Concentration Of Ions In An Electrolyte Solution Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). There is direct relation between ion concentration and the current density limit, caused by ion diffusion. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. When ionic compounds dissolve. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.numerade.com
SOLVEDCalculate the concentration of all ions pr… Increasing The Concentration Of Ions In An Electrolyte Solution An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.toppr.com
Electrolytes when dissolved in water dissociate into their constituent Increasing The Concentration Of Ions In An Electrolyte Solution The larger the concentration of ions, the better the solutions. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). In the limiting state, all. When ionic compounds dissolve in water, the ions in the solid separate. Increasing The Concentration Of Ions In An Electrolyte Solution.
From webmis.highland.cc.il.us
Electrolysis Increasing The Concentration Of Ions In An Electrolyte Solution An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. There is direct relation between ion concentration and the current density limit, caused by ion diffusion. Solutions may also conduct electricity if they contain dissolved ions, with. Increasing The Concentration Of Ions In An Electrolyte Solution.
From courses.lumenlearning.com
Electrolyte Balance Anatomy and Physiology II Increasing The Concentration Of Ions In An Electrolyte Solution An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). In the limiting state, all. There is direct relation between ion concentration and the current density limit, caused by ion diffusion. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Solutions may also conduct electricity if. Increasing The Concentration Of Ions In An Electrolyte Solution.
From www.researchgate.net
Effect of electrolyte concentration on the production of hydrogen gas Increasing The Concentration Of Ions In An Electrolyte Solution Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : In the limiting state, all. Solutions may also. Increasing The Concentration Of Ions In An Electrolyte Solution.
From slideplayer.com
Section 4.5 Concentrations of Solutions ppt download Increasing The Concentration Of Ions In An Electrolyte Solution In the limiting state, all. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 9.02:_the_solution_process : Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion. Increasing The Concentration Of Ions In An Electrolyte Solution.