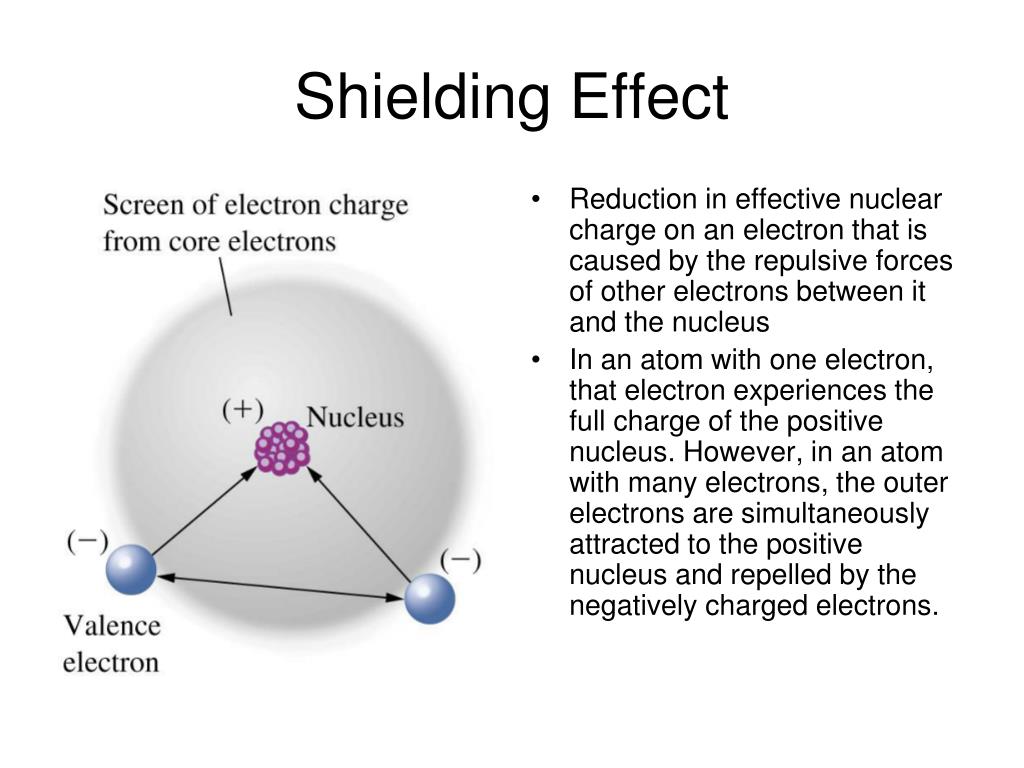
Which Electrons Are Shielding Electrons . Electrons in an \ (s\) orbital can shield \. H is the only atom in which shielding does not occur. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): Shielding occurs in all atoms and ions that have more than one electron.
from www.slideserve.com
Shielding occurs in all atoms and ions that have more than one electron. H is the only atom in which shielding does not occur. Electrons in an \ (s\) orbital can shield \. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2):
PPT Periodic Trends PowerPoint Presentation, free download ID3652853
Which Electrons Are Shielding Electrons H is the only atom in which shielding does not occur. Electrons in an \ (s\) orbital can shield \. Shielding occurs in all atoms and ions that have more than one electron. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): H is the only atom in which shielding does not occur. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision Which Electrons Are Shielding Electrons Shielding occurs in all atoms and ions that have more than one electron. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): H is the only atom in which shielding does not occur. However,. Which Electrons Are Shielding Electrons.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick Which Electrons Are Shielding Electrons H is the only atom in which shielding does not occur. Electrons in an \ (s\) orbital can shield \. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience. Which Electrons Are Shielding Electrons.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free Which Electrons Are Shielding Electrons Shielding occurs in all atoms and ions that have more than one electron. Electrons in an \ (s\) orbital can shield \. H is the only atom in which shielding does not occur. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. The electron configuration of li. Which Electrons Are Shielding Electrons.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID2836425 Which Electrons Are Shielding Electrons Electrons in an \ (s\) orbital can shield \. H is the only atom in which shielding does not occur. Shielding occurs in all atoms and ions that have more than one electron. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. The electron configuration of li. Which Electrons Are Shielding Electrons.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick Which Electrons Are Shielding Electrons However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. Shielding occurs in all atoms and ions that have more than one electron. Electrons in an \ (s\) orbital can shield \. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does. Which Electrons Are Shielding Electrons.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube Which Electrons Are Shielding Electrons Electrons in an \ (s\) orbital can shield \. Shielding occurs in all atoms and ions that have more than one electron. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does. Which Electrons Are Shielding Electrons.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint Which Electrons Are Shielding Electrons The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): Electrons in an \ (s\) orbital can shield \. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive. Which Electrons Are Shielding Electrons.
From slideplayer.com
Coulomb’s Law PERIODIC TRENDS Nuclear Charge Electron Shielding ppt Which Electrons Are Shielding Electrons H is the only atom in which shielding does not occur. Electrons in an \ (s\) orbital can shield \. Shielding occurs in all atoms and ions that have more than one electron. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. The electron configuration of li. Which Electrons Are Shielding Electrons.
From ar.inspiredpencil.com
Electron Shielding Which Electrons Are Shielding Electrons H is the only atom in which shielding does not occur. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): Shielding occurs in all atoms and ions that have more than one electron. Electrons. Which Electrons Are Shielding Electrons.
From wisc.pb.unizin.org
M7Q8 Core and Valence Electrons, Shielding, Zeff Chem 103/104 Which Electrons Are Shielding Electrons However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): H is the. Which Electrons Are Shielding Electrons.
From www.slideserve.com
PPT Notes One Unit Nine Chapter Four PowerPoint Presentation, free Which Electrons Are Shielding Electrons The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): H is the only atom in which shielding does not occur. However, the presence of inner electrons between the nucleus and the outer electrons creates. Which Electrons Are Shielding Electrons.
From ar.inspiredpencil.com
Electron Shielding Which Electrons Are Shielding Electrons However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): Shielding occurs in. Which Electrons Are Shielding Electrons.
From www.slideserve.com
PPT Chapter 6 The Periodic Table PowerPoint Presentation, free Which Electrons Are Shielding Electrons The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): Electrons in an \ (s\) orbital can shield \. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive. Which Electrons Are Shielding Electrons.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID Which Electrons Are Shielding Electrons The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): H is the only atom in which shielding does not occur. However, the presence of inner electrons between the nucleus and the outer electrons creates. Which Electrons Are Shielding Electrons.
From www.youtube.com
Electron shielding YouTube Which Electrons Are Shielding Electrons The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): H is the only atom in which shielding does not occur. However, the presence of inner electrons between the nucleus and the outer electrons creates. Which Electrons Are Shielding Electrons.
From www.slideserve.com
PPT Unit 5 The Periodic Table PowerPoint Presentation, free download Which Electrons Are Shielding Electrons The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): Shielding occurs in all atoms and ions that have more than one electron. Electrons in an \ (s\) orbital can shield \. However, the presence. Which Electrons Are Shielding Electrons.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity Which Electrons Are Shielding Electrons Electrons in an \ (s\) orbital can shield \. Shielding occurs in all atoms and ions that have more than one electron. H is the only atom in which shielding does not occur. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the. Which Electrons Are Shielding Electrons.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation, free download Which Electrons Are Shielding Electrons Shielding occurs in all atoms and ions that have more than one electron. H is the only atom in which shielding does not occur. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): Electrons. Which Electrons Are Shielding Electrons.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding Which Electrons Are Shielding Electrons However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. Shielding occurs in all atoms and ions that have more than one electron. Electrons in an \ (s\) orbital can shield \. H is the only atom in which shielding does not occur. The electron configuration of li. Which Electrons Are Shielding Electrons.
From www.shemmassianconsulting.com
Atoms and Periodic Trends for the MCAT Everything You Need to Know Which Electrons Are Shielding Electrons H is the only atom in which shielding does not occur. Shielding occurs in all atoms and ions that have more than one electron. Electrons in an \ (s\) orbital can shield \. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. The electron configuration of li. Which Electrons Are Shielding Electrons.
From slideplayer.com
History of the Periodic Table ppt download Which Electrons Are Shielding Electrons Shielding occurs in all atoms and ions that have more than one electron. H is the only atom in which shielding does not occur. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. Electrons in an \ (s\) orbital can shield \. The electron configuration of li. Which Electrons Are Shielding Electrons.
From www.slideserve.com
PPT The Development of the Periodic Table PowerPoint Presentation Which Electrons Are Shielding Electrons H is the only atom in which shielding does not occur. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. Electrons in an \ (s\) orbital can shield \. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience. Which Electrons Are Shielding Electrons.
From socratic.org
How are shielding effect and atomic radius related? Socratic Which Electrons Are Shielding Electrons However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. Electrons in an \ (s\) orbital can shield \. Shielding occurs in all atoms and ions that have more than one electron. H is the only atom in which shielding does not occur. The electron configuration of li. Which Electrons Are Shielding Electrons.
From slideplayer.com
Development of the Periodic Table ppt download Which Electrons Are Shielding Electrons However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): Shielding occurs in. Which Electrons Are Shielding Electrons.
From surfguppy.com
What is Electronegativity? Which Electrons Are Shielding Electrons Electrons in an \ (s\) orbital can shield \. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. Shielding occurs in all atoms and ions that have more than one electron. H is the only atom in which shielding does not occur. The electron configuration of li. Which Electrons Are Shielding Electrons.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive Which Electrons Are Shielding Electrons Electrons in an \ (s\) orbital can shield \. Shielding occurs in all atoms and ions that have more than one electron. H is the only atom in which shielding does not occur. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the. Which Electrons Are Shielding Electrons.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID3652853 Which Electrons Are Shielding Electrons Shielding occurs in all atoms and ions that have more than one electron. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): Electrons in an \ (s\) orbital can shield \. H is the. Which Electrons Are Shielding Electrons.
From slideplayer.com
Periodic Trends Chemistry I. ppt download Which Electrons Are Shielding Electrons Electrons in an \ (s\) orbital can shield \. H is the only atom in which shielding does not occur. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience. Which Electrons Are Shielding Electrons.
From www.slideserve.com
PPT Chapter 6 Periodic Table PowerPoint Presentation, free download Which Electrons Are Shielding Electrons H is the only atom in which shielding does not occur. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. Shielding occurs in all atoms and ions that have more than one electron. Electrons in an \ (s\) orbital can shield \. The electron configuration of li. Which Electrons Are Shielding Electrons.
From www.breakingatom.com
Shielding Which Electrons Are Shielding Electrons Electrons in an \ (s\) orbital can shield \. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. Shielding occurs in all atoms and ions that have more than one electron. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does. Which Electrons Are Shielding Electrons.
From quizizz.com
Chemistry Periodic Law and Electron Shielding Quiz Quizizz Which Electrons Are Shielding Electrons H is the only atom in which shielding does not occur. Shielding occurs in all atoms and ions that have more than one electron. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. Electrons in an \ (s\) orbital can shield \. The electron configuration of li. Which Electrons Are Shielding Electrons.
From slideplayer.com
Elemental Properties and Patterns ppt download Which Electrons Are Shielding Electrons H is the only atom in which shielding does not occur. However, the presence of inner electrons between the nucleus and the outer electrons creates a repulsive force that shields the outer electrons. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the. Which Electrons Are Shielding Electrons.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID3646736 Which Electrons Are Shielding Electrons H is the only atom in which shielding does not occur. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): Electrons in an \ (s\) orbital can shield \. Shielding occurs in all atoms. Which Electrons Are Shielding Electrons.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.3.1 Electronegativity翰林国际教育 Which Electrons Are Shielding Electrons The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): H is the only atom in which shielding does not occur. Electrons in an \ (s\) orbital can shield \. Shielding occurs in all atoms. Which Electrons Are Shielding Electrons.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 Which Electrons Are Shielding Electrons H is the only atom in which shielding does not occur. Electrons in an \ (s\) orbital can shield \. The electron configuration of li is 1s 2 2s 1, and the 1s valence electron does not experience the 3+ charge of the nucleus because of the shielding by the two core electrons (2s 2): However, the presence of inner. Which Electrons Are Shielding Electrons.