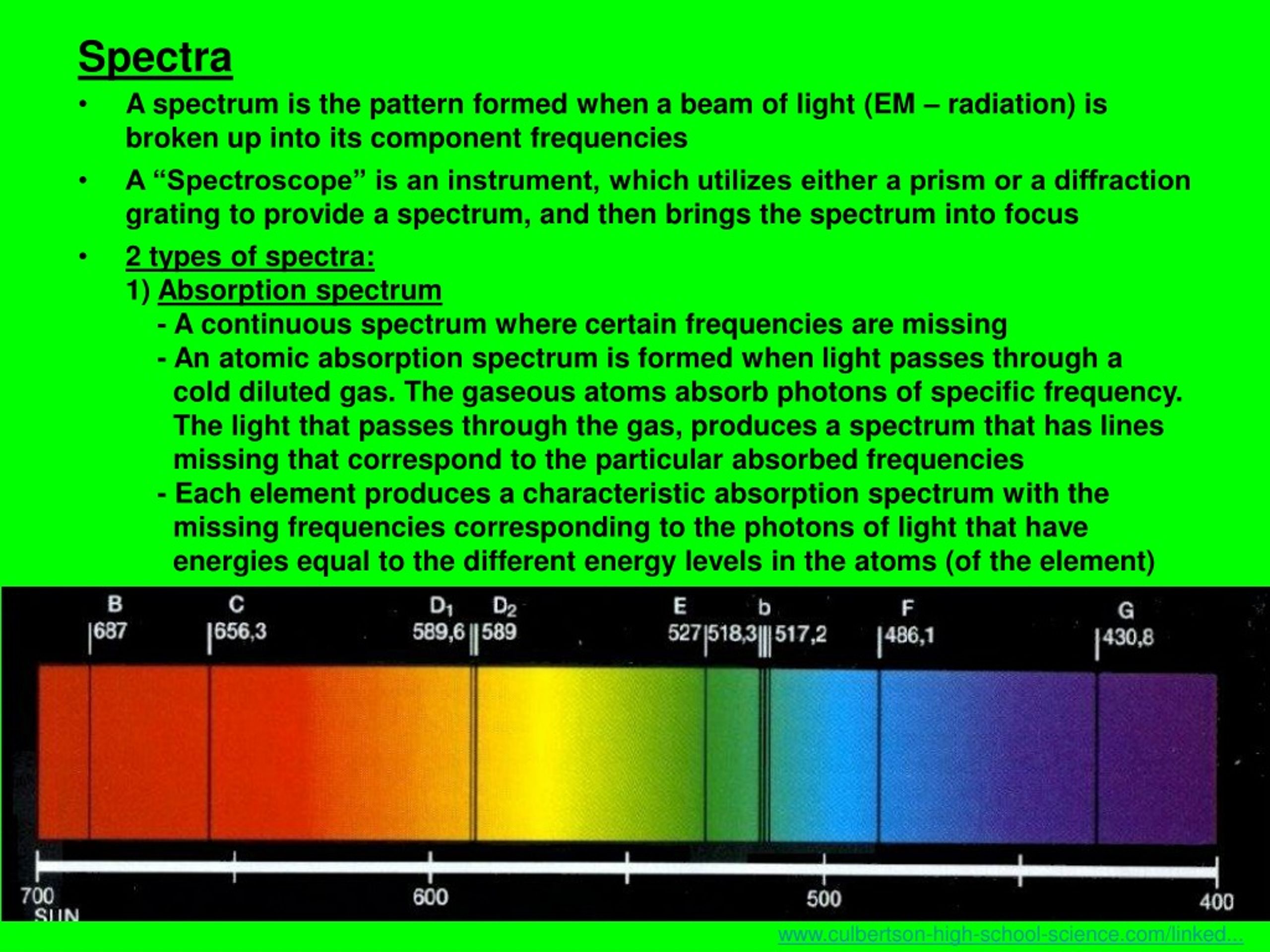
Emission Spectra Definition Chemistry Simple . An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an atom. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current.
from www.slideserve.com
In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an atom. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The actual wavelengths of the lines are predictably.
PPT EMISSION AND ABSORPTION SPECTRA PowerPoint Presentation, free download ID361475
Emission Spectra Definition Chemistry Simple In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The actual wavelengths of the lines are predictably. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an atom. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample.
From www.youtube.com
Emission and absorption spectra YouTube Emission Spectra Definition Chemistry Simple In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different. Emission Spectra Definition Chemistry Simple.
From www.acs.org
Spectrum American Chemical Society Emission Spectra Definition Chemistry Simple In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The actual wavelengths of the lines are predictably. An emission spectrum is the characteristic pattern of wavelengths. Emission Spectra Definition Chemistry Simple.
From classnotes.org.in
Absorption and Emission Spectra Chemistry, Class 11, Structure Of Atom Emission Spectra Definition Chemistry Simple In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Atomic emission spectroscopy (aes) is an analytical technique used. Emission Spectra Definition Chemistry Simple.
From www.youtube.com
Spectrum Basic Introduction YouTube Emission Spectra Definition Chemistry Simple An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Atomic emission spectroscopy (aes) is an analytical technique used. Emission Spectra Definition Chemistry Simple.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Emission Spectra Definition Chemistry Simple In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to. Emission Spectra Definition Chemistry Simple.
From sekaangry.weebly.com
Atomic emission spectrum chemistry definition sekaangry Emission Spectra Definition Chemistry Simple An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. In chemistry, an emission spectrum refers to the range of wavelengths. Emission Spectra Definition Chemistry Simple.
From www.slideserve.com
PPT PreIB/PreAP CHEMISTRY PowerPoint Presentation, free download ID1597831 Emission Spectra Definition Chemistry Simple Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by. Emission Spectra Definition Chemistry Simple.
From www.pinterest.com
Atomic Emission Spectrum Easy Science Chemistry basics, Electron configuration Emission Spectra Definition Chemistry Simple An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are predictably. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The spectroscope separates the light of. Emission Spectra Definition Chemistry Simple.
From mungfali.com
Atomic Emission Spectrum Of Elements Emission Spectra Definition Chemistry Simple The actual wavelengths of the lines are predictably. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. An atomic emission spectrum is the pattern of lines. Emission Spectra Definition Chemistry Simple.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Emission Spectra Definition Chemistry Simple The actual wavelengths of the lines are predictably. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An atomic emission spectrum is the pattern of lines formed when light passes through. Emission Spectra Definition Chemistry Simple.
From www.studypool.com
SOLUTION 20201012023722atomic Emission Spectra Studypool Emission Spectra Definition Chemistry Simple An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an atom. The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. In. Emission Spectra Definition Chemistry Simple.
From montessorimuddle.org
Emission Spectra How Atoms Emit and Absorb Light Montessori Muddle Emission Spectra Definition Chemistry Simple The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an atom. An. Emission Spectra Definition Chemistry Simple.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Presentation ID5901440 Emission Spectra Definition Chemistry Simple In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The spectroscope separates the light of varying wavelengths and. Emission Spectra Definition Chemistry Simple.
From rightmetal.weebly.com
Atomic emission spectrum chemistry definition rightmetal Emission Spectra Definition Chemistry Simple In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are predictably. Atomic. Emission Spectra Definition Chemistry Simple.
From www.researchgate.net
Emission spectra of RTILI and II and the acidic and basic forms of... Download Scientific Diagram Emission Spectra Definition Chemistry Simple In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The spectroscope separates the light of varying wavelengths and. Emission Spectra Definition Chemistry Simple.
From terrence-kgonzales.blogspot.com
Describe the Emission Spectrum of Hydrogen Ib Chemistry Emission Spectra Definition Chemistry Simple An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an atom. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom. Emission Spectra Definition Chemistry Simple.
From saylordotorg.github.io
Atomic Spectra and Models of the Atom Emission Spectra Definition Chemistry Simple Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an. Emission Spectra Definition Chemistry Simple.
From www.slideserve.com
PPT Electrons and Periodicity PowerPoint Presentation, free download ID1587154 Emission Spectra Definition Chemistry Simple The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an atom. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The actual wavelengths of the lines are predictably. An. Emission Spectra Definition Chemistry Simple.
From www.nagwa.com
Lesson Video Emission and Absorption Spectra Nagwa Emission Spectra Definition Chemistry Simple An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The actual wavelengths of the lines are predictably. An. Emission Spectra Definition Chemistry Simple.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Spectra Definition Chemistry Simple In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The actual wavelengths of the lines are predictably. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. An atomic emission spectrum is the pattern of lines. Emission Spectra Definition Chemistry Simple.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Definition Chemistry Simple The spectroscope separates the light of varying wavelengths and we observe a line spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different. Emission Spectra Definition Chemistry Simple.
From www.slideserve.com
PPT CHEMISTRY XL14A NATURE OF LIGHT AND THE ATOM PowerPoint Presentation ID6599431 Emission Spectra Definition Chemistry Simple An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an atom. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric. Emission Spectra Definition Chemistry Simple.
From imagine.gsfc.nasa.gov
Spectra Introduction Emission Spectra Definition Chemistry Simple The spectroscope separates the light of varying wavelengths and we observe a line spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an atom. An atomic emission spectrum. Emission Spectra Definition Chemistry Simple.
From chem.libretexts.org
10.1 Overview of Spectroscopy Chemistry LibreTexts Emission Spectra Definition Chemistry Simple An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an atom. The actual wavelengths of the lines are predictably. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom. Emission Spectra Definition Chemistry Simple.
From chem.libretexts.org
6.3 Line Spectra and the Bohr Model Chemistry LibreTexts Emission Spectra Definition Chemistry Simple The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an atom. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The. Emission Spectra Definition Chemistry Simple.
From byjus.com
Atomic Spectra (Emission Spectrum & Absorption Spectra) Detailed Explanation with Videos Emission Spectra Definition Chemistry Simple Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The actual wavelengths of the lines are predictably. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an atom. An atomic emission spectrum is the pattern of lines formed when light passes through a. Emission Spectra Definition Chemistry Simple.
From www.priyamstudycentre.com
Spectroscopy Definition, Types, Applications Emission Spectra Definition Chemistry Simple Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The actual wavelengths of the lines are predictably. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. An atomic emission spectrum is the pattern of lines. Emission Spectra Definition Chemistry Simple.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID6041161 Emission Spectra Definition Chemistry Simple Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an. Emission Spectra Definition Chemistry Simple.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/104 Resource Book Emission Spectra Definition Chemistry Simple The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an atom. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into. Emission Spectra Definition Chemistry Simple.
From www.slideserve.com
PPT EMISSION AND ABSORPTION SPECTRA PowerPoint Presentation, free download ID361475 Emission Spectra Definition Chemistry Simple An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are predictably. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. In chemistry, an emission spectrum refers. Emission Spectra Definition Chemistry Simple.
From testbook.com
Emission Spectrum Definition, Types, Atomic Spectra of Hydrogen Emission Spectra Definition Chemistry Simple The actual wavelengths of the lines are predictably. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. An. Emission Spectra Definition Chemistry Simple.
From www.researchgate.net
Normalized excitation and fluorescence emission spectra of fluorescent... Download Scientific Emission Spectra Definition Chemistry Simple The actual wavelengths of the lines are predictably. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by. Emission Spectra Definition Chemistry Simple.
From manuallistcantabank.z21.web.core.windows.net
A Diagram Of The Spectrum Emission Spectra Definition Chemistry Simple In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to. Emission Spectra Definition Chemistry Simple.
From www.toppr.com
Define emission spectron and absorption spectrum. Emission Spectra Definition Chemistry Simple An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an atom. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom. Emission Spectra Definition Chemistry Simple.
From quizzfullmarks77.z19.web.core.windows.net
Atomic Emission Spectrum Definition Chemistry Emission Spectra Definition Chemistry Simple An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation emitted by an atom. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom. Emission Spectra Definition Chemistry Simple.