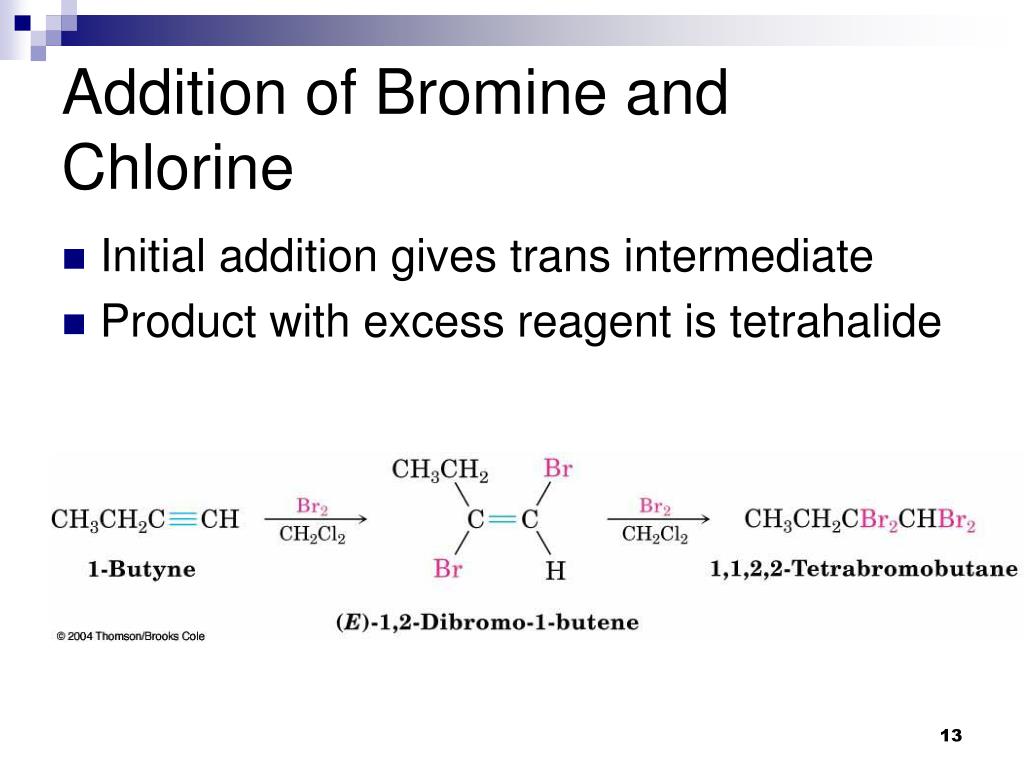
Bromine And Chlorine Bond . The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. The most stable oxidation state of the element is −1, in. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. The bromine reagent is in reddish color, and. Bromine is less reactive, means it. The halogens are located on the left of the noble gases on the periodic table. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this. Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily as chlorine does.
from www.slideserve.com
The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this. The most stable oxidation state of the element is −1, in. Bromine is less reactive, means it. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The halogens are located on the left of the noble gases on the periodic table. Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily as chlorine does. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. The bromine reagent is in reddish color, and.
PPT Alkynes An Introduction to Organic Synthesis PowerPoint
Bromine And Chlorine Bond Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this. Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily as chlorine does. The halogens are located on the left of the noble gases on the periodic table. Bromine is less reactive, means it. The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this. The bromine reagent is in reddish color, and. The most stable oxidation state of the element is −1, in. The relative lower reactivity of bromine makes it exhibits a much greater selectivity.
From slideplayer.com
Covalent and Ionic Bonding ppt download Bromine And Chlorine Bond The halogens are located on the left of the noble gases on the periodic table. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Bromine is less reactive, means it. The most stable oxidation state of the element is −1, in. Ionic bonds are. Bromine And Chlorine Bond.
From wisc.pb.unizin.org
Resonance Structures and Formal Charge (M8Q3) UWMadison Chemistry Bromine And Chlorine Bond Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily as chlorine does. The most stable oxidation state of the element is −1, in. The halogens are located on the left of the noble gases on the periodic table. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. The reaction between. Bromine And Chlorine Bond.
From www.slideserve.com
PPT Chapter Twenty PowerPoint Presentation, free download ID6301153 Bromine And Chlorine Bond The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The halogens are located on the left of the noble gases on the periodic table. The most stable oxidation state of the element is −1, in. Ionic bonds are electrostatic forces of attraction, that is,. Bromine And Chlorine Bond.
From www.chegg.com
Solved Alkanes react with chlorine and bromine in the Bromine And Chlorine Bond The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this. Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily. Bromine And Chlorine Bond.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Bromine And Chlorine Bond Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily as chlorine does. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this. The halogens are located on the left of the noble gases on the periodic table. Bromine is less reactive, means it.. Bromine And Chlorine Bond.
From resource.studiaacademy.com
Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Bromine And Chlorine Bond Bromine is less reactive, means it. The most stable oxidation state of the element is −1, in. The halogens are located on the left of the noble gases on the periodic table. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. The bromine reagent is in reddish color, and. The reaction between c=c double bond and. Bromine And Chlorine Bond.
From www.studocu.com
Chapter 5 1 Overview of Addition Reactions 2 Addition of Bromine and Bromine And Chlorine Bond Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The most stable oxidation state of the element is −1, in. Bromine displaces hydrogen. Bromine And Chlorine Bond.
From www.slideserve.com
PPT Alkynes An Introduction to Organic Synthesis PowerPoint Bromine And Chlorine Bond The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this. Bromine is less reactive, means it. The halogens are located on the left of the. Bromine And Chlorine Bond.
From www.doheny.com
Bromine vs. Chlorine Everything You Need to Know Bromine And Chlorine Bond The bromine reagent is in reddish color, and. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between. Bromine And Chlorine Bond.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Bromine And Chlorine Bond The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in reddish color, and. Bromine is less reactive, means it. The halogens are located on the left of the noble gases on the periodic table. Bromine displaces hydrogen from saturated hydrocarbons. Bromine And Chlorine Bond.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Bromine And Chlorine Bond The halogens are located on the left of the noble gases on the periodic table. The most stable oxidation state of the element is −1, in. Bromine is less reactive, means it. The bromine reagent is in reddish color, and. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of. Bromine And Chlorine Bond.
From www.differencebetween.com
Difference Between Bromine and Chlorine Compare the Difference Bromine And Chlorine Bond The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. The most stable oxidation state of the element is −1, in. Bromine is less reactive, means it. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. The reaction between c=c double bond. Bromine And Chlorine Bond.
From chem.libretexts.org
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Bromine And Chlorine Bond Bromine is less reactive, means it. The bromine reagent is in reddish color, and. The most stable oxidation state of the element is −1, in. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this. The. Bromine And Chlorine Bond.
From edu.rsc.org
Reactions of chlorine, bromine and iodine with aluminium Experiment Bromine And Chlorine Bond The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this. The most stable oxidation state of the element is −1, in. The reaction between c=c. Bromine And Chlorine Bond.
From www.science-revision.co.uk
Electrophilic addition Bromine And Chlorine Bond Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily as chlorine does. Bromine is less reactive, means it. The halogens are located on the left of the noble gases on the periodic table. The most stable oxidation state of the element is −1, in. Ionic bonds are electrostatic forces of attraction, that is, the. Bromine And Chlorine Bond.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison Bromine And Chlorine Bond The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this. The. Bromine And Chlorine Bond.
From slideplayer.com
Chapter 5 Alkene Addition Reactions ppt download Bromine And Chlorine Bond The most stable oxidation state of the element is −1, in. Bromine is less reactive, means it. The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge. Bromine And Chlorine Bond.
From resource.studiaacademy.com
2.2 Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Bromine And Chlorine Bond The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Bromine is less reactive, means it. Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily as chlorine does. Ionic bonds are electrostatic forces of attraction, that is, the attractive. Bromine And Chlorine Bond.
From www.alamy.com
Fluorine, Bromine and Chlorine Molecular Model of Atom. Vector Bromine And Chlorine Bond Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily as chlorine does. Bromine is less reactive, means it. The halogens are located on the left of the noble gases on the periodic table. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this.. Bromine And Chlorine Bond.
From www.numerade.com
SOLVED Classify each of the following bonds as ionic, polar covalent Bromine And Chlorine Bond The most stable oxidation state of the element is −1, in. Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily as chlorine does. The halogens are located on the left of the noble gases on the periodic table. The bromine reagent is in reddish color, and. The chemical properties of bromine are similar to. Bromine And Chlorine Bond.
From chem.libretexts.org
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Bromine And Chlorine Bond The bromine reagent is in reddish color, and. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this. The most stable oxidation state of the element is −1, in. The halogens are located on the left of the noble gases on the periodic table. Bromine displaces hydrogen from saturated. Bromine And Chlorine Bond.
From stock.adobe.com
Vecteur Stock Diatomic molecules diagram shows elements that exist as Bromine And Chlorine Bond The halogens are located on the left of the noble gases on the periodic table. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this. The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than.. Bromine And Chlorine Bond.
From www.slideserve.com
PPT Chapter 9 Alkynes An Introduction to Organic Synthesis Bromine And Chlorine Bond Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily as chlorine does. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. The halogens are located on the left of the noble gases on the periodic table. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between. Bromine And Chlorine Bond.
From fyorzqilq.blob.core.windows.net
Bromine And Chlorine Ionic Equation at Kelly McFadden blog Bromine And Chlorine Bond The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. Ionic bonds are electrostatic forces of attraction, that is, the attractive. Bromine And Chlorine Bond.
From www.researchgate.net
Patterns of the molecular ion with multiple chlorine or bromine atoms Bromine And Chlorine Bond The bromine reagent is in reddish color, and. The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. The halogens are located on the left of the noble gases on the periodic table.. Bromine And Chlorine Bond.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Bromine And Chlorine Bond The relative lower reactivity of bromine makes it exhibits a much greater selectivity. The most stable oxidation state of the element is −1, in. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Ionic bonds are electrostatic forces of attraction, that is, the attractive. Bromine And Chlorine Bond.
From pediaa.com
Difference Between Bromine and Chlorine Bromine And Chlorine Bond The most stable oxidation state of the element is −1, in. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Bromine displaces hydrogen. Bromine And Chlorine Bond.
From www.slideserve.com
PPT Types of Bonding and Lewis Structures PowerPoint Presentation Bromine And Chlorine Bond The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. Bromine is less reactive, means it. The bromine reagent is in reddish color, and. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in. Bromine And Chlorine Bond.
From derekcarrsavvy-chemist.blogspot.co.uk
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Bromine And Chlorine Bond The halogens are located on the left of the noble gases on the periodic table. The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this.. Bromine And Chlorine Bond.
From fyoosnzjx.blob.core.windows.net
Bromine And Chloride Ion Reaction at Gwendolyn Veltri blog Bromine And Chlorine Bond Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily as chlorine does. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this. The reaction between c=c double bond and bromine. Bromine And Chlorine Bond.
From material-properties.org
Chlorine and Bromine Comparison Properties Material Properties Bromine And Chlorine Bond The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The most stable oxidation state of the element is −1, in.. Bromine And Chlorine Bond.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points Bromine And Chlorine Bond The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily as chlorine does. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical. Bromine And Chlorine Bond.
From www.differencebetween.net
Difference Between Bromine and Chlorine Difference Between Bromine And Chlorine Bond The halogens are located on the left of the noble gases on the periodic table. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily as chlorine does. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between. Bromine And Chlorine Bond.
From slideplayer.com
Chapter 6 Chemical Bonding. ppt download Bromine And Chlorine Bond The halogens are located on the left of the noble gases on the periodic table. The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. The bromine reagent is in reddish color, and. The reaction between c=c double bond and bromine (br 2) can be used. Bromine And Chlorine Bond.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Bromine And Chlorine Bond The most stable oxidation state of the element is −1, in. The chemical properties of bromine are similar to those of chlorine, although bromine is the weaker oxidizing agent and its reactivity is less than. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. The reaction between c=c double bond and bromine (br 2) can be. Bromine And Chlorine Bond.