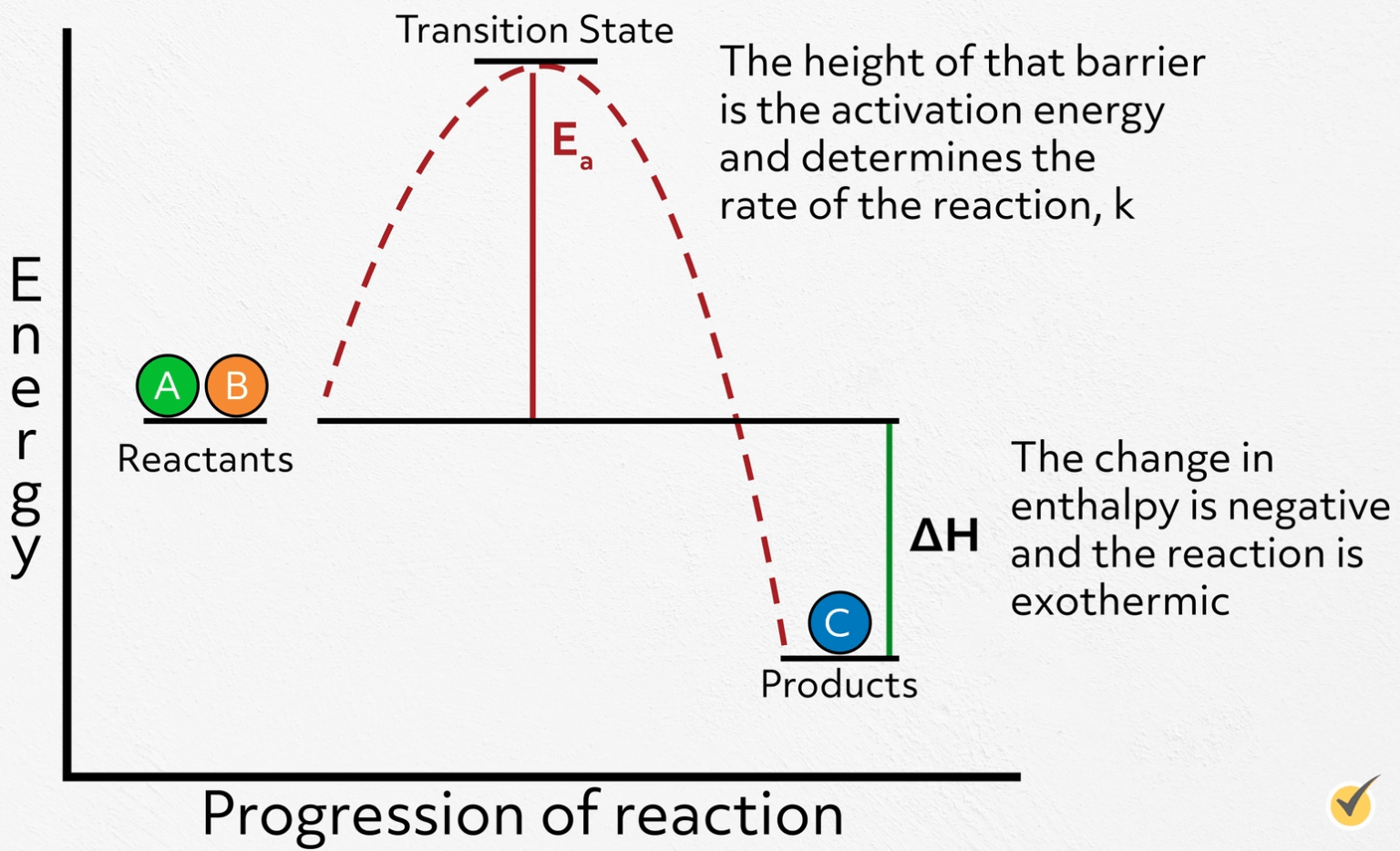
Catalysts Slow Down Chemical Reactions . A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. In some cases, chemists wish to speed up reactions that are too slow or slow down reactions that are too fast. When the reaction has finished, the mass of. A more energetic particle which didn't happen to collide successfully and produce a reaction, could find itself slowed down an instant later as a result of a collision. Students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of hydrogen peroxide. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. Catalysts are substances that can speed up or slow down chemical reactions. In order to gain any control. They can do this without being used up or chemically changed. In the absence of contaminants this reaction is very slow, but a variety of substances, ranging from iodine, metal oxides, trace amount of.
from www.mometrix.com
In a heterogeneous reaction, the catalyst is in a different phase from the reactants. In the absence of contaminants this reaction is very slow, but a variety of substances, ranging from iodine, metal oxides, trace amount of. A more energetic particle which didn't happen to collide successfully and produce a reaction, could find itself slowed down an instant later as a result of a collision. When the reaction has finished, the mass of. In order to gain any control. Catalysts are substances that can speed up or slow down chemical reactions. Students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of hydrogen peroxide. They can do this without being used up or chemically changed. In some cases, chemists wish to speed up reactions that are too slow or slow down reactions that are too fast. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end.
What is a Catalyst? Chemistry Review (Video)
Catalysts Slow Down Chemical Reactions They can do this without being used up or chemically changed. In the absence of contaminants this reaction is very slow, but a variety of substances, ranging from iodine, metal oxides, trace amount of. In order to gain any control. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. They can do this without being used up or chemically changed. In some cases, chemists wish to speed up reactions that are too slow or slow down reactions that are too fast. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. A more energetic particle which didn't happen to collide successfully and produce a reaction, could find itself slowed down an instant later as a result of a collision. Catalysts are substances that can speed up or slow down chemical reactions. When the reaction has finished, the mass of. Students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of hydrogen peroxide.
From study.com
Effect of Catalysts on Rates of Reaction Lesson Catalysts Slow Down Chemical Reactions In some cases, chemists wish to speed up reactions that are too slow or slow down reactions that are too fast. Students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of hydrogen peroxide. In a heterogeneous reaction, the catalyst is in a different phase from the reactants.. Catalysts Slow Down Chemical Reactions.
From pt.slideshare.net
Rates of chemical reactions Catalysts Slow Down Chemical Reactions Students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of hydrogen peroxide. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. They can do this without being used up or chemically changed. A more energetic particle which didn't happen to collide successfully. Catalysts Slow Down Chemical Reactions.
From www.slideserve.com
PPT Rates of Reactions PowerPoint Presentation, free download ID266964 Catalysts Slow Down Chemical Reactions Catalysts are substances that can speed up or slow down chemical reactions. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. A more energetic particle which didn't happen to collide successfully and produce a reaction, could find itself slowed down an instant later as a result of a collision. They can do. Catalysts Slow Down Chemical Reactions.
From owlcation.com
Chemical Reactions and Chemical Equations Owlcation Catalysts Slow Down Chemical Reactions Catalysts are substances that can speed up or slow down chemical reactions. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. They can do this without being used up or chemically changed. When the reaction has finished, the mass of. Students watch a video and do a quick activity to see that. Catalysts Slow Down Chemical Reactions.
From 2012books.lardbucket.org
Catalysis Catalysts Slow Down Chemical Reactions In the absence of contaminants this reaction is very slow, but a variety of substances, ranging from iodine, metal oxides, trace amount of. When the reaction has finished, the mass of. In order to gain any control. A more energetic particle which didn't happen to collide successfully and produce a reaction, could find itself slowed down an instant later as. Catalysts Slow Down Chemical Reactions.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Catalysts Slow Down Chemical Reactions In the absence of contaminants this reaction is very slow, but a variety of substances, ranging from iodine, metal oxides, trace amount of. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. In some cases, chemists wish to speed up reactions that are too slow or slow down reactions that are too. Catalysts Slow Down Chemical Reactions.
From www.chegg.com
Solved Part C Catalyst and the rate of chemical reaction Catalysts Slow Down Chemical Reactions They can do this without being used up or chemically changed. In order to gain any control. When the reaction has finished, the mass of. In the absence of contaminants this reaction is very slow, but a variety of substances, ranging from iodine, metal oxides, trace amount of. In a heterogeneous reaction, the catalyst is in a different phase from. Catalysts Slow Down Chemical Reactions.
From techschems.com
Unlocking the Secrets of Enzyme Catalysis through the Lock and Key Diagram Catalysts Slow Down Chemical Reactions Catalysts are substances that can speed up or slow down chemical reactions. In order to gain any control. They can do this without being used up or chemically changed. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. When the reaction has finished, the mass of. In the absence of contaminants this reaction is very. Catalysts Slow Down Chemical Reactions.
From lessonlibminimalist.z13.web.core.windows.net
Chemical Reaction In Physical Science Catalysts Slow Down Chemical Reactions They can do this without being used up or chemically changed. In the absence of contaminants this reaction is very slow, but a variety of substances, ranging from iodine, metal oxides, trace amount of. Students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of hydrogen peroxide. In. Catalysts Slow Down Chemical Reactions.
From ppt-online.org
Rates of reaction презентация онлайн Catalysts Slow Down Chemical Reactions They can do this without being used up or chemically changed. In some cases, chemists wish to speed up reactions that are too slow or slow down reactions that are too fast. Catalysts are substances that can speed up or slow down chemical reactions. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. When the. Catalysts Slow Down Chemical Reactions.
From exowzdqkr.blob.core.windows.net
Enzymes Do Chemical Reaction at Anna Schofield blog Catalysts Slow Down Chemical Reactions In some cases, chemists wish to speed up reactions that are too slow or slow down reactions that are too fast. In order to gain any control. In the absence of contaminants this reaction is very slow, but a variety of substances, ranging from iodine, metal oxides, trace amount of. Students watch a video and do a quick activity to. Catalysts Slow Down Chemical Reactions.
From www.sridianti.com
Enzyme Catalysis Mechanisms, Functions, and RealWorld Examples www Catalysts Slow Down Chemical Reactions Students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of hydrogen peroxide. In order to gain any control. In some cases, chemists wish to speed up reactions that are too slow or slow down reactions that are too fast. When the reaction has finished, the mass of.. Catalysts Slow Down Chemical Reactions.
From slideplayer.com
Reviewing Main Ideas Chemical Changes ppt download Catalysts Slow Down Chemical Reactions A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. They can do this without being used up or chemically changed. Catalysts are substances that can speed up or slow down chemical reactions. In order to gain any control. In a heterogeneous reaction, the catalyst is in a different phase from the reactants.. Catalysts Slow Down Chemical Reactions.
From digital-library.theiet.org
Influence of the reaction time on the physicalchemical and catalytic Catalysts Slow Down Chemical Reactions Students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of hydrogen peroxide. In some cases, chemists wish to speed up reactions that are too slow or slow down reactions that are too fast. They can do this without being used up or chemically changed. A catalyst is. Catalysts Slow Down Chemical Reactions.
From shapeguidance1.gitlab.io
Outrageous Does A Catalyst Increase The Rate Of Reaction Year 12 Catalysts Slow Down Chemical Reactions A more energetic particle which didn't happen to collide successfully and produce a reaction, could find itself slowed down an instant later as a result of a collision. They can do this without being used up or chemically changed. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. Catalysts are substances that can speed up. Catalysts Slow Down Chemical Reactions.
From slideplayer.com
Catalysis Gold oxidation catalyst ppt download Catalysts Slow Down Chemical Reactions A more energetic particle which didn't happen to collide successfully and produce a reaction, could find itself slowed down an instant later as a result of a collision. In the absence of contaminants this reaction is very slow, but a variety of substances, ranging from iodine, metal oxides, trace amount of. They can do this without being used up or. Catalysts Slow Down Chemical Reactions.
From slideplayer.com
Radioisotopes Introduction to Nuclear Chemistry ppt download Catalysts Slow Down Chemical Reactions In order to gain any control. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. When the reaction has finished, the mass of. In some cases, chemists wish to speed up reactions that are too slow or slow down reactions that are too fast. A catalyst is a substance which speeds up a reaction, but. Catalysts Slow Down Chemical Reactions.
From www.nagwa.com
Question Video Identifying the Reason Why Catalysts Are Used in Catalysts Slow Down Chemical Reactions Catalysts are substances that can speed up or slow down chemical reactions. When the reaction has finished, the mass of. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. In order to gain any control. Students watch a video and do a quick activity to see that a catalyst can increase the rate of the. Catalysts Slow Down Chemical Reactions.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Catalysts Slow Down Chemical Reactions When the reaction has finished, the mass of. Students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of hydrogen peroxide. A more energetic particle which didn't happen to collide successfully and produce a reaction, could find itself slowed down an instant later as a result of a. Catalysts Slow Down Chemical Reactions.
From www.slideserve.com
PPT Chymosin Lab PowerPoint Presentation ID484941 Catalysts Slow Down Chemical Reactions Catalysts are substances that can speed up or slow down chemical reactions. A more energetic particle which didn't happen to collide successfully and produce a reaction, could find itself slowed down an instant later as a result of a collision. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. In the absence of contaminants this. Catalysts Slow Down Chemical Reactions.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalysts Slow Down Chemical Reactions When the reaction has finished, the mass of. In the absence of contaminants this reaction is very slow, but a variety of substances, ranging from iodine, metal oxides, trace amount of. In order to gain any control. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. A more energetic particle which didn't. Catalysts Slow Down Chemical Reactions.
From www.pinterest.com
The catalyst can change the speed of a reaction without being used up Catalysts Slow Down Chemical Reactions In some cases, chemists wish to speed up reactions that are too slow or slow down reactions that are too fast. In the absence of contaminants this reaction is very slow, but a variety of substances, ranging from iodine, metal oxides, trace amount of. Catalysts are substances that can speed up or slow down chemical reactions. In a heterogeneous reaction,. Catalysts Slow Down Chemical Reactions.
From en.ppt-online.org
Rates of reaction online presentation Catalysts Slow Down Chemical Reactions A more energetic particle which didn't happen to collide successfully and produce a reaction, could find itself slowed down an instant later as a result of a collision. In the absence of contaminants this reaction is very slow, but a variety of substances, ranging from iodine, metal oxides, trace amount of. In some cases, chemists wish to speed up reactions. Catalysts Slow Down Chemical Reactions.
From www.vedantu.com
A catalyst increases the rate of reaction by……A. Decreasing enthalpyB Catalysts Slow Down Chemical Reactions When the reaction has finished, the mass of. In the absence of contaminants this reaction is very slow, but a variety of substances, ranging from iodine, metal oxides, trace amount of. Catalysts are substances that can speed up or slow down chemical reactions. In some cases, chemists wish to speed up reactions that are too slow or slow down reactions. Catalysts Slow Down Chemical Reactions.
From www.emokykla.lt
Indukuoto atitikmens modelis Catalysts Slow Down Chemical Reactions Catalysts are substances that can speed up or slow down chemical reactions. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. They can do this without being used up or chemically changed. A more energetic particle which didn't happen to collide successfully and produce a reaction, could find itself slowed down an instant later as. Catalysts Slow Down Chemical Reactions.
From slideplayer.com
Unit Nuclear Chemistry ppt download Catalysts Slow Down Chemical Reactions In the absence of contaminants this reaction is very slow, but a variety of substances, ranging from iodine, metal oxides, trace amount of. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. Catalysts are substances that can speed up or slow down chemical reactions. In some cases, chemists wish to speed up reactions that are. Catalysts Slow Down Chemical Reactions.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalysts Slow Down Chemical Reactions In some cases, chemists wish to speed up reactions that are too slow or slow down reactions that are too fast. When the reaction has finished, the mass of. Catalysts are substances that can speed up or slow down chemical reactions. In order to gain any control. In the absence of contaminants this reaction is very slow, but a variety. Catalysts Slow Down Chemical Reactions.
From lessoncampusunspelt.z13.web.core.windows.net
Activity Of Enzyme Formula Catalysts Slow Down Chemical Reactions A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. In some cases, chemists wish to speed up reactions that are too slow or slow down reactions that are too fast. When the reaction has finished, the mass of. In a heterogeneous reaction, the catalyst is in a different phase from the reactants.. Catalysts Slow Down Chemical Reactions.
From beta.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalysts Slow Down Chemical Reactions In order to gain any control. In some cases, chemists wish to speed up reactions that are too slow or slow down reactions that are too fast. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. Students watch a video and do a quick activity to see that a catalyst can increase. Catalysts Slow Down Chemical Reactions.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii Catalysts Slow Down Chemical Reactions Catalysts are substances that can speed up or slow down chemical reactions. In some cases, chemists wish to speed up reactions that are too slow or slow down reactions that are too fast. In the absence of contaminants this reaction is very slow, but a variety of substances, ranging from iodine, metal oxides, trace amount of. A catalyst is a. Catalysts Slow Down Chemical Reactions.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Catalysts Slow Down Chemical Reactions A more energetic particle which didn't happen to collide successfully and produce a reaction, could find itself slowed down an instant later as a result of a collision. When the reaction has finished, the mass of. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. They can do this without being used up or chemically. Catalysts Slow Down Chemical Reactions.
From ppt-online.org
Rates of reaction презентация онлайн Catalysts Slow Down Chemical Reactions When the reaction has finished, the mass of. Students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of hydrogen peroxide. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. In the absence of contaminants this reaction is very slow,. Catalysts Slow Down Chemical Reactions.