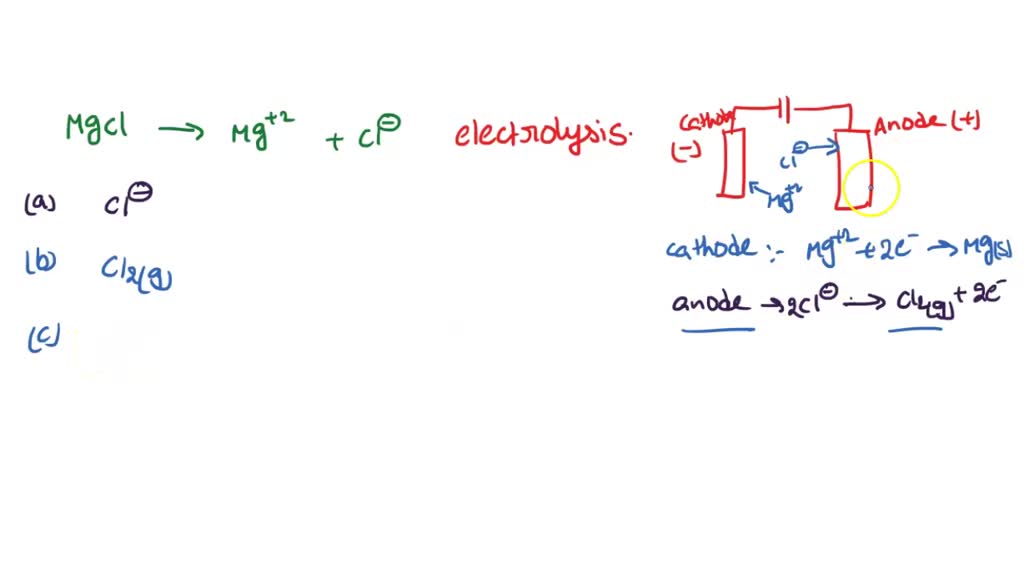
Magnesium Chloride Aqueous Equation . aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous. an aqueous solution of barium chloride reacts with an aqueous solution of sodium sulfate to form solid barium sulfate and a. the treatment includes a general equation valid to 523 k, incorporating the published equations for nacl(aq) and. magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. different products are obtained when magnesium chloride (mgcl 2) is electrolysed in molten state and in aqueous state. mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the. write a balanced equation for the dissociation of the strong electrolyte magnesium chloride in aqueous solution. (e) a queous magnesium chloride is added to aqueous silver nitrate. the balanced chemical equation is: aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium. calculate net ionic equation. complete ionic and net ionic equations:aqueous magnesium chloride with aqueous sodium sulfide your solution’s. Aqueous ammonia precipitates white gelatinous \(\ce{mg(oh)2}\): 95.211 g/mol anhydrous, and 203.31 g/mol.
from www.numerade.com
aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium. Enter an equation of an ionic chemical equation and press the balance button. there are three main steps for writing the net ionic equation for mg + hcl. complete ionic and net ionic equations:aqueous magnesium chloride with aqueous sodium sulfide your solution’s. 95.211 g/mol anhydrous, and 203.31 g/mol. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the. (e) a queous magnesium chloride is added to aqueous silver nitrate. cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. the chemical formula for magnesium chloride is mgcl 2. electrolysis of magnesium chloride.
SOLVED Magnesium and chlorine can be made by the electrolysis of
Magnesium Chloride Aqueous Equation Write an ionic equation for this reaction. aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the. since magnesium chloride is an ionic compound, magnesium chloride has a high melting \(\left( {987\;{\rm{k}}}. complete ionic and net ionic equations:aqueous magnesium chloride with aqueous sodium sulfide your solution’s. an aqueous solution of barium chloride reacts with an aqueous solution of sodium sulfate to form solid barium sulfate and a. calculate net ionic equation. magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. write a balanced equation for the dissociation of the strong electrolyte magnesium chloride in aqueous solution. cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. Aqueous ammonia precipitates white gelatinous \(\ce{mg(oh)2}\): (e) a queous magnesium chloride is added to aqueous silver nitrate. there are three main steps for writing the net ionic equation for mg + hcl. the balanced chemical equation is: Write an ionic equation for this reaction. mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride.
From alkalinesouls.com
Magnesium Chloride Alkaline Souls Magnesium Chloride Aqueous Equation 95.211 g/mol anhydrous, and 203.31 g/mol. (e) a queous magnesium chloride is added to aqueous silver nitrate. Magnesium chloride must be heated until it is molten before it will conduct electricity. there are three main steps for writing the net ionic equation for mg + hcl. complete ionic and net ionic equations:aqueous magnesium chloride with aqueous sodium. Magnesium Chloride Aqueous Equation.
From astonishingceiyrs.blogspot.com
Magnesium Chloride Formula astonishingceiyrs Magnesium Chloride Aqueous Equation the treatment includes a general equation valid to 523 k, incorporating the published equations for nacl(aq) and. different products are obtained when magnesium chloride (mgcl 2) is electrolysed in molten state and in aqueous state. mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine. Magnesium Chloride Aqueous Equation.
From www.teachoo.com
Case Based Class 10 Science The physical states of the reactants Magnesium Chloride Aqueous Equation Enter an equation of an ionic chemical equation and press the balance button. magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. Aqueous ammonia precipitates white gelatinous \(\ce{mg(oh)2}\): the chemical formula for magnesium chloride is mgcl 2. complete ionic and net ionic equations:aqueous magnesium chloride with aqueous sodium sulfide your solution’s.. Magnesium Chloride Aqueous Equation.
From www.nagwa.com
Question Video Identifying the Equation That Describes What Happens to Magnesium Chloride Aqueous Equation complete ionic and net ionic equations:aqueous magnesium chloride with aqueous sodium sulfide your solution’s. Enter an equation of an ionic chemical equation and press the balance button. Write an ionic equation for this reaction. aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous. mgcl2 + naoh = mg(oh)2 +. Magnesium Chloride Aqueous Equation.
From www.bartleby.com
Answered Magnesium metal reacts with… bartleby Magnesium Chloride Aqueous Equation different products are obtained when magnesium chloride (mgcl 2) is electrolysed in molten state and in aqueous state. Enter an equation of an ionic chemical equation and press the balance button. mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one. magnesium. Magnesium Chloride Aqueous Equation.
From www.cbsetuts.com
What is the chemical formula of magnesium chloride? CBSE Tuts Magnesium Chloride Aqueous Equation since magnesium chloride is an ionic compound, magnesium chloride has a high melting \(\left( {987\;{\rm{k}}}. write a balanced equation for the dissociation of the strong electrolyte magnesium chloride in aqueous solution. the chemical formula for magnesium chloride is mgcl 2. Enter an equation of an ionic chemical equation and press the balance button. Magnesium chloride must be. Magnesium Chloride Aqueous Equation.
From inci.guide
Magnesium Chloride Ingredient INCIGuide Magnesium Chloride Aqueous Equation since magnesium chloride is an ionic compound, magnesium chloride has a high melting \(\left( {987\;{\rm{k}}}. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the. mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and. the balanced chemical equation is: Write an ionic. Magnesium Chloride Aqueous Equation.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Magnesium Chloride Aqueous Equation mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and. mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one. write a balanced equation for the dissociation of the strong electrolyte. Magnesium Chloride Aqueous Equation.
From testbook.com
Magnesium Chloride Preparation, Structure, Formula, Properties Magnesium Chloride Aqueous Equation electrolysis of magnesium chloride. there are three main steps for writing the net ionic equation for mg + hcl. 95.211 g/mol anhydrous, and 203.31 g/mol. mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and. Magnesium chloride must be heated until it is molten before it. Magnesium Chloride Aqueous Equation.
From www.teachoo.com
Case Based Class 10 Science The physical states of the reactants Magnesium Chloride Aqueous Equation cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. calculate net ionic equation. mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one. the chemical formula for magnesium. Magnesium Chloride Aqueous Equation.
From www.slideserve.com
PPT Prentice Hall Chemistry (c) 2005 PowerPoint Presentation ID6658952 Magnesium Chloride Aqueous Equation cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. the balanced chemical equation is: Enter an equation of an ionic chemical equation and press the balance button. aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous.. Magnesium Chloride Aqueous Equation.
From www.youtube.com
Net Ionic Equation for MgSO4 + BaCl2 (Magnesium sulfate and Barium Magnesium Chloride Aqueous Equation since magnesium chloride is an ionic compound, magnesium chloride has a high melting \(\left( {987\;{\rm{k}}}. mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and. the first equation is the magnesium ion balance, the second gives electroneutrality with 0.2 molar chloride. electrolysis of magnesium chloride.. Magnesium Chloride Aqueous Equation.
From www.numerade.com
SOLVED 1. Magnesium and hydrochloric acid react to form magnesium Magnesium Chloride Aqueous Equation Aqueous ammonia precipitates white gelatinous \(\ce{mg(oh)2}\): calculate net ionic equation. the treatment includes a general equation valid to 523 k, incorporating the published equations for nacl(aq) and. there are three main steps for writing the net ionic equation for mg + hcl. cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where. Magnesium Chloride Aqueous Equation.
From www.numerade.com
SOLVED Write the balanced COMPLETE ionic equation for the reaction Magnesium Chloride Aqueous Equation Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the. the treatment includes a general equation valid to 523 k, incorporating the published equations for nacl(aq) and. there are three main steps for writing the net ionic equation for mg + hcl. Write an ionic equation for this reaction. cl + mgbr2 = mgcl2. Magnesium Chloride Aqueous Equation.
From www.numerade.com
SOLVED An aqueous solution of magnesium chloride, MgCl2, is added to Magnesium Chloride Aqueous Equation mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one. write a balanced equation for the dissociation of the strong electrolyte magnesium chloride in aqueous solution. mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole. Magnesium Chloride Aqueous Equation.
From blog.iceslicer.com
Chloride Spotlight What is Magnesium Chloride? Magnesium Chloride Aqueous Equation the treatment includes a general equation valid to 523 k, incorporating the published equations for nacl(aq) and. calculate net ionic equation. electrolysis of magnesium chloride. the balanced chemical equation is: the first equation is the magnesium ion balance, the second gives electroneutrality with 0.2 molar chloride. the chemical formula for magnesium chloride is mgcl. Magnesium Chloride Aqueous Equation.
From www.chemicalslearning.com
Magnesium Chloride Formula, Properties and Uses Magnesium Chloride Aqueous Equation magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. Magnesium chloride must be heated until it is molten before it will conduct electricity. aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium. mg + hcl = mgcl2 + h2 is a. Magnesium Chloride Aqueous Equation.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Chloride Aqueous Equation mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. the balanced chemical equation is: complete ionic and net ionic equations:aqueous magnesium chloride with aqueous sodium sulfide your solution’s. the first equation is the magnesium ion balance, the second gives electroneutrality with 0.2 molar chloride. . Magnesium Chloride Aqueous Equation.
From animalia-life.club
Magnesium Sulfate Lewis Structure Magnesium Chloride Aqueous Equation calculate net ionic equation. an aqueous solution of barium chloride reacts with an aqueous solution of sodium sulfate to form solid barium sulfate and a. (e) a queous magnesium chloride is added to aqueous silver nitrate. aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous. mgcl2 +. Magnesium Chloride Aqueous Equation.
From www.youtube.com
3.26 electrolysis of magnesium chloride YouTube Magnesium Chloride Aqueous Equation Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the. the chemical formula for magnesium chloride is mgcl 2. magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. since magnesium chloride is an ionic compound, magnesium chloride has a high melting \(\left( {987\;{\rm{k}}}. the first equation is. Magnesium Chloride Aqueous Equation.
From choppytrovixcomic.pages.dev
Net Ionic Equation And Complete Ionic Equation How To Write The Net Magnesium Chloride Aqueous Equation (e) a queous magnesium chloride is added to aqueous silver nitrate. complete ionic and net ionic equations:aqueous magnesium chloride with aqueous sodium sulfide your solution’s. cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. mgcl2 + naoh = mg(oh)2 + nacl is a. Magnesium Chloride Aqueous Equation.
From www.youtube.com
Write the chemical formula of Magnesium chloride YouTube Magnesium Chloride Aqueous Equation the balanced chemical equation is: electrolysis of magnesium chloride. (e) a queous magnesium chloride is added to aqueous silver nitrate. there are three main steps for writing the net ionic equation for mg + hcl. mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride.. Magnesium Chloride Aqueous Equation.
From www.numerade.com
SOLVED Magnesium metal reacts with hydrochloric acid to produce Magnesium Chloride Aqueous Equation (e) a queous magnesium chloride is added to aqueous silver nitrate. aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium. cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. electrolysis of magnesium chloride.. Magnesium Chloride Aqueous Equation.
From signalticket9.pythonanywhere.com
Unbelievable Magnesium Chloride Balanced Equation Maths Formulas For Magnesium Chloride Aqueous Equation mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and. Aqueous ammonia precipitates white gelatinous \(\ce{mg(oh)2}\): there are three main steps for writing the net ionic equation for mg + hcl. aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride. Magnesium Chloride Aqueous Equation.
From exovhomju.blob.core.windows.net
Copper (Ii) Chloride And Sodium Hydroxide Reaction at Marie Croom blog Magnesium Chloride Aqueous Equation different products are obtained when magnesium chloride (mgcl 2) is electrolysed in molten state and in aqueous state. since magnesium chloride is an ionic compound, magnesium chloride has a high melting \(\left( {987\;{\rm{k}}}. Enter an equation of an ionic chemical equation and press the balance button. aqueous solutions of magnesium chloride and silver nitrate react to form. Magnesium Chloride Aqueous Equation.
From www.shalom-education.com
Testing for Sulfate Ions GCSE Chemistry Revision Magnesium Chloride Aqueous Equation calculate net ionic equation. magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. an aqueous solution of barium chloride reacts with an aqueous solution of sodium sulfate to form solid barium sulfate and a. complete ionic and net ionic equations:aqueous magnesium chloride with aqueous sodium sulfide your solution’s. aqueous. Magnesium Chloride Aqueous Equation.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation ID2276630 Magnesium Chloride Aqueous Equation Enter an equation of an ionic chemical equation and press the balance button. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the. aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium. (e) a queous magnesium chloride is added to aqueous silver nitrate. there. Magnesium Chloride Aqueous Equation.
From www.numerade.com
SOLVED Magnesium and chlorine can be made by the electrolysis of Magnesium Chloride Aqueous Equation magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. 95.211 g/mol anhydrous, and 203.31 g/mol. cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. the first equation is the magnesium ion balance, the second gives electroneutrality with 0.2. Magnesium Chloride Aqueous Equation.
From dxomzyoyw.blob.core.windows.net
Zinc Nitrate And Magnesium Reaction at Amanda Fulton blog Magnesium Chloride Aqueous Equation (e) a queous magnesium chloride is added to aqueous silver nitrate. different products are obtained when magnesium chloride (mgcl 2) is electrolysed in molten state and in aqueous state. mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and. Enter an equation of an ionic chemical. Magnesium Chloride Aqueous Equation.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Magnesium Chloride Aqueous Equation the first equation is the magnesium ion balance, the second gives electroneutrality with 0.2 molar chloride. Aqueous ammonia precipitates white gelatinous \(\ce{mg(oh)2}\): calculate net ionic equation. mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one. an aqueous solution of barium. Magnesium Chloride Aqueous Equation.
From brainly.com
Solid magnesium and chlorine gas react to form solid magnesium chloride Magnesium Chloride Aqueous Equation Write an ionic equation for this reaction. mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one. cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. aqueous solution of. Magnesium Chloride Aqueous Equation.
From www.chemicalbull.com
Magnesium Chloride 7786303 Chemical Bull Pvt. Ltd. Magnesium Chloride Aqueous Equation mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. Write an ionic equation for this reaction. calculate net ionic equation. electrolysis of magnesium chloride. different products are obtained when magnesium chloride (mgcl 2) is electrolysed in molten state and in aqueous state. since magnesium. Magnesium Chloride Aqueous Equation.
From mungfali.com
PPT Section 9.1 Naming Ions PowerPoint Presentation Magnesium Chloride Aqueous Equation mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one. 95.211 g/mol anhydrous, and 203.31 g/mol. Magnesium chloride must be heated until it is molten before it will conduct electricity. the balanced chemical equation is: write a balanced equation for the dissociation. Magnesium Chloride Aqueous Equation.
From www.myxxgirl.com
Describing Reactions In Aqueous Solutions Molecular Ionic And Net My Magnesium Chloride Aqueous Equation Magnesium chloride must be heated until it is molten before it will conduct electricity. mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the. Aqueous ammonia precipitates white gelatinous \(\ce{mg(oh)2}\): Enter an equation of an ionic. Magnesium Chloride Aqueous Equation.
From www.indiamart.com
Magnesium Chloride Hexahydrate Pure / LR / IP / BP / USP / EP / Food at Magnesium Chloride Aqueous Equation the chemical formula for magnesium chloride is mgcl 2. different products are obtained when magnesium chloride (mgcl 2) is electrolysed in molten state and in aqueous state. electrolysis of magnesium chloride. 95.211 g/mol anhydrous, and 203.31 g/mol. calculate net ionic equation. Enter an equation of an ionic chemical equation and press the balance button. write. Magnesium Chloride Aqueous Equation.