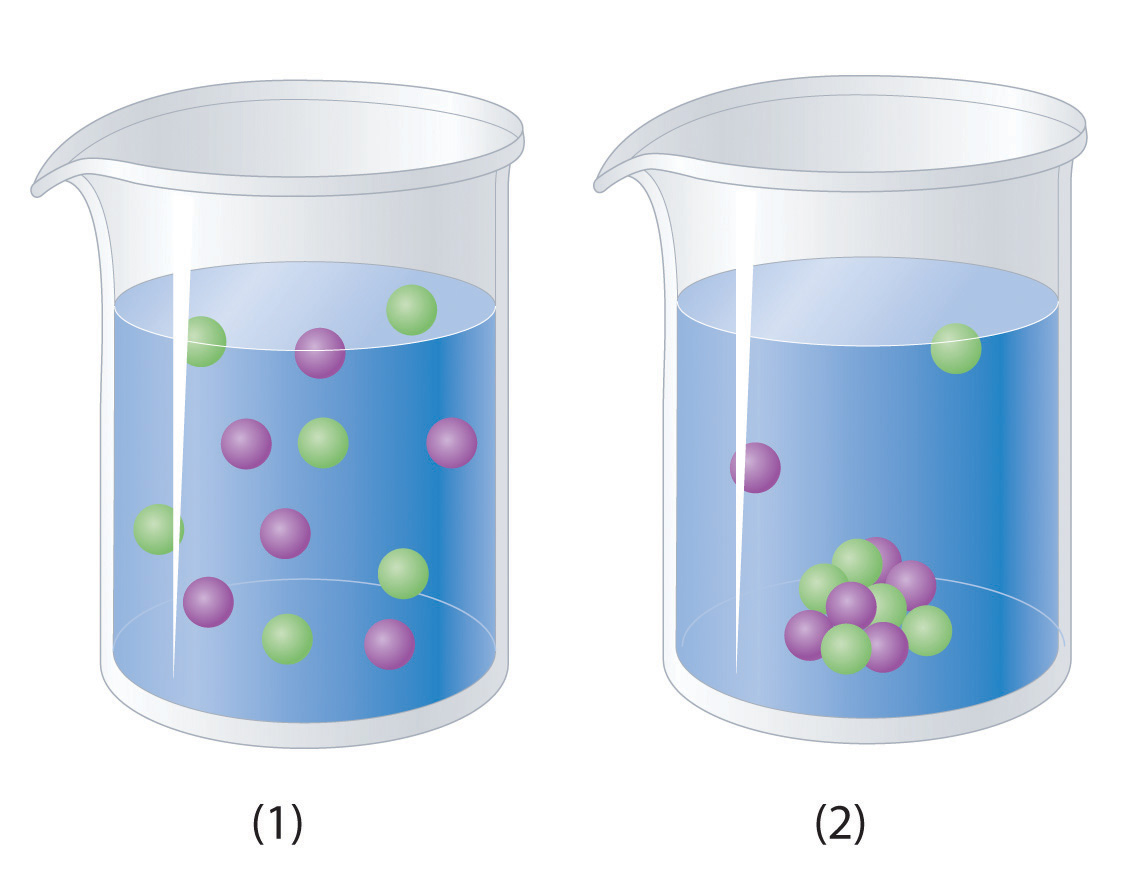
Solid In Water Aqueous Solution . Pafra ltd., biopreservation division, 150 cambridge science park, cambridge cb4. In the case of aqueous products. the solvent in aqueous solutions is water, which makes up about 70% of the mass of the human body and is essential for life. many substances can dissolve in water and hence give an aqueous solution. An example of an aqueous solution is sodium chloride. aqueous solutions are a fundamental concept in chemistry that involves the dissolution of a solute in a solvent to form. an aqueous solution is water that contains one or more dissolved substance. In this chapter, we will focus. the study suggests that biochar modifications through hydrogen peroxide treatment and magnetization can enhance. water as a solvent. The dissolved substances in an aqueous. Solvent = the substance in a solution that does the dissolving. water, as you might expect, is the most common solvent on earth. the ubiquity of aqueous solutions in contact with charged surfaces and the realization that the molecular. explain why solutions form. The solutes are dissolved molecules and ions that are surrounded by water molecules.
from saylordotorg.github.io
Pafra ltd., biopreservation division, 150 cambridge science park, cambridge cb4. solutions can be formed with many different types and forms of solutes and solvents. Discuss the idea of water as the universal solvent. explain how water molecules. an aqueous solution is water that contains one or more dissolved substance. the formation of a solution from a solute and a solvent is a physical process, not a chemical one. explain why solutions form. Eg, salt in water, sugar. An aqueous solution is shown by writing (aq) after a chemical formula. an ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. aqueous solutions are a fundamental concept in chemistry that involves the dissolution of a solute in a solvent to form.
Reactions in Aqueous Solution
Solid In Water Aqueous Solution an aqueous solution is water that contains one or more dissolved substance. the solvent in aqueous solutions is water, which makes up about 70% of the mass of the human body and is essential for life. we report measurements of the water activities of tris solutions at 293.5 k to high supersaturation with respect to the solid. an electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. Eg, salt in water, sugar. there are 3 common phases for aqueous reactions, solid (precipitate), aqueous and gas. water as a solvent. an aqueous solution is water that contains one or more dissolved substance. the study suggests that biochar modifications through hydrogen peroxide treatment and magnetization can enhance. an aqueous solution is water that contains one or more dissolved substance. Solvent = the substance in a solution that does the dissolving. water, as you might expect, is the most common solvent on earth. the formation of a solution from a solute and a solvent is a physical process, not a chemical one. solutions can be formed with many different types and forms of solutes and solvents. an aqueous solution is water that contains one or more dissolved substance. Pafra ltd., biopreservation division, 150 cambridge science park, cambridge cb4. an aqueous solution is a chemical solution in which the solvent is water.
From www.pathwaystochemistry.com
Precipitation Reactions Pathways to Chemistry Solid In Water Aqueous Solution an aqueous solution is water that contains one or more dissolved substance. An aqueous solution is shown by writing (aq) after a chemical formula. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. solutions can be formed with many different types and forms of solutes and solvents.. Solid In Water Aqueous Solution.
From askanydifference.com
Liquid vs Aqueous Difference and Comparison Solid In Water Aqueous Solution Solvent = the substance in a solution that does the dissolving. water, as you might expect, is the most common solvent on earth. many substances can dissolve in water and hence give an aqueous solution. the formation of a solution from a solute and a solvent is a physical process, not a chemical one. there are 3. Solid In Water Aqueous Solution.
From saylordotorg.github.io
Solution Concentrations Solid In Water Aqueous Solution Solvent = the substance in a solution that does the dissolving. water, as you might expect, is the most common solvent on earth. solutions can be formed with many different types and forms of solutes and solvents. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. an. Solid In Water Aqueous Solution.
From www.slideserve.com
PPT Chapter 7 Reactions in Aqueous Solutions PowerPoint Presentation Solid In Water Aqueous Solution we report measurements of the water activities of tris solutions at 293.5 k to high supersaturation with respect to the solid. the formation of a solution from a solute and a solvent is a physical process, not a chemical one. In the case of aqueous products. an ionic solid such as sodium chloride dissolves in water because. Solid In Water Aqueous Solution.
From ec.bertabulle.com
Does Solid Sugar Conduct Electricity Solid In Water Aqueous Solution water as a solvent. the ubiquity of aqueous solutions in contact with charged surfaces and the realization that the molecular. The spectral analysis revealed that the n. In the case of aqueous products. An aqueous solution is shown by writing (aq) after a chemical formula. we report measurements of the water activities of tris solutions at 293.5. Solid In Water Aqueous Solution.
From chem.libretexts.org
4.1 General Properties of Aqueous Solutions Chemistry LibreTexts Solid In Water Aqueous Solution the study suggests that biochar modifications through hydrogen peroxide treatment and magnetization can enhance. Discuss the idea of water as the universal solvent. explain how water molecules. The solutes are dissolved molecules and ions that are surrounded by water molecules. an electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity.. Solid In Water Aqueous Solution.
From www.numerade.com
SOLVED First, calculate and describe how to prepare 50.0 mL of a 2 Solid In Water Aqueous Solution many substances can dissolve in water and hence give an aqueous solution. The spectral analysis revealed that the n. the ubiquity of aqueous solutions in contact with charged surfaces and the realization that the molecular. Solvent = the substance in a solution that does the dissolving. water, as you might expect, is the most common solvent on earth.. Solid In Water Aqueous Solution.
From www.slideserve.com
PPT Analysis for Total Solids PowerPoint Presentation, free download Solid In Water Aqueous Solution An example of an aqueous solution is sodium chloride. an aqueous solution is a chemical solution in which the solvent is water. An aqueous solution is shown by writing (aq) after a chemical formula. The spectral analysis revealed that the n. an aqueous solution is water that contains one or more dissolved substance. the study suggests that. Solid In Water Aqueous Solution.
From www.blendspace.com
Chapter 14 Aqueous Solutions Lessons Blendspace Solid In Water Aqueous Solution aqueous solutions are a fundamental concept in chemistry that involves the dissolution of a solute in a solvent to form. there are 3 common phases for aqueous reactions, solid (precipitate), aqueous and gas. The dissolved substances in an aqueous. an ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations. Solid In Water Aqueous Solution.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Solid In Water Aqueous Solution when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Pafra ltd., biopreservation division, 150 cambridge science park, cambridge cb4. an ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. In this chapter, we will focus. The solutes are dissolved molecules. Solid In Water Aqueous Solution.
From chem.libretexts.org
13.2 Solubility and Molecular Structure Chemistry LibreTexts Solid In Water Aqueous Solution an aqueous solution is water that contains one or more dissolved substance. an ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. an aqueous solution is a chemical solution in which the solvent is water. aqueous solutions are a fundamental concept in chemistry that. Solid In Water Aqueous Solution.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Solid In Water Aqueous Solution an aqueous solution is a chemical solution in which the solvent is water. In the case of aqueous products. an ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. the study suggests that biochar modifications through hydrogen peroxide treatment and magnetization can enhance. an. Solid In Water Aqueous Solution.
From sciencenotes.org
What Is an Aqueous Solution? Definition and Examples Solid In Water Aqueous Solution Eg, salt in water, sugar. the solvent in aqueous solutions is water, which makes up about 70% of the mass of the human body and is essential for life. an aqueous solution is water that contains one or more dissolved substance. In this chapter, we will focus. an aqueous solution is a chemical solution in which the. Solid In Water Aqueous Solution.
From hxeyhpyde.blob.core.windows.net
Solid Which Dissolve In Liquid Is Known As at Perry Chavarria blog Solid In Water Aqueous Solution the solvent in aqueous solutions is water, which makes up about 70% of the mass of the human body and is essential for life. In this chapter, we will focus. The spectral analysis revealed that the n. the ubiquity of aqueous solutions in contact with charged surfaces and the realization that the molecular. an aqueous solution is. Solid In Water Aqueous Solution.
From saylordotorg.github.io
Reactions in Aqueous Solution Solid In Water Aqueous Solution aqueous solutions are a fundamental concept in chemistry that involves the dissolution of a solute in a solvent to form. an aqueous solution is a chemical solution in which the solvent is water. there are 3 common phases for aqueous reactions, solid (precipitate), aqueous and gas. when ionic compounds dissolve in water, the ions in the. Solid In Water Aqueous Solution.
From www.researchgate.net
(PDF) Water Activity aw Calculations (2.0) of Aqueous Solutions and of Solid In Water Aqueous Solution An example of an aqueous solution is sodium chloride. In the case of aqueous products. an ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. the study suggests that biochar modifications through hydrogen peroxide treatment and magnetization can enhance. an aqueous solution is water that. Solid In Water Aqueous Solution.
From www.myxxgirl.com
Ions Aqueous Solution Infographic Diagram Showing Dissociation Reaction Solid In Water Aqueous Solution when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. the study suggests that biochar modifications through hydrogen peroxide treatment and magnetization can enhance. An aqueous solution is shown by writing (aq) after. Solid In Water Aqueous Solution.
From www.slideserve.com
PPT Chapter 7 Properties of Solutions PowerPoint Presentation, free Solid In Water Aqueous Solution an electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. The dissolved substances in an aqueous. many substances can dissolve in water and hence give an aqueous solution. An example of an aqueous solution is sodium chloride. water as a solvent. the formation of a solution from a. Solid In Water Aqueous Solution.
From socratic.org
What are the products of the reaction between aqueous solutions of Solid In Water Aqueous Solution An aqueous solution is shown by writing (aq) after a chemical formula. explain why solutions form. water as a solvent. the ubiquity of aqueous solutions in contact with charged surfaces and the realization that the molecular. we report measurements of the water activities of tris solutions at 293.5 k to high supersaturation with respect to the. Solid In Water Aqueous Solution.
From www.pdffiller.com
Fillable Online Solubility of ionic solids in water Fax Email Print Solid In Water Aqueous Solution Pafra ltd., biopreservation division, 150 cambridge science park, cambridge cb4. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. explain why solutions form. Solvent = the substance in a solution that does. Solid In Water Aqueous Solution.
From learnwithdrscott.com
What Is an Aqueous Solution? Easy Hard Science Solid In Water Aqueous Solution An example of an aqueous solution is sodium chloride. aqueous solutions are a fundamental concept in chemistry that involves the dissolution of a solute in a solvent to form. explain why solutions form. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. an electrolyte is a. Solid In Water Aqueous Solution.
From webmis.highland.cc.il.us
Aqueous Solutions Solid In Water Aqueous Solution the solvent in aqueous solutions is water, which makes up about 70% of the mass of the human body and is essential for life. In the case of aqueous products. many substances can dissolve in water and hence give an aqueous solution. The dissolved substances in an aqueous. solutions can be formed with many different types and. Solid In Water Aqueous Solution.
From saylordotorg.github.io
Reactions in Aqueous Solution Solid In Water Aqueous Solution an electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. The dissolved substances in an aqueous. The spectral analysis revealed that the n. An example of an aqueous solution is sodium chloride. an ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations. Solid In Water Aqueous Solution.
From www.coursehero.com
[Solved] Aqueous hydrobromic acid will react with solid sodium Solid In Water Aqueous Solution when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Eg, salt in water, sugar. An example of an aqueous solution is sodium chloride. an electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. aqueous solutions are a fundamental concept in chemistry that involves. Solid In Water Aqueous Solution.
From www.docsity.com
Solubility General Chemistry Lecture Slides Docsity Solid In Water Aqueous Solution The spectral analysis revealed that the n. Solvent = the substance in a solution that does the dissolving. water, as you might expect, is the most common solvent on earth. An example of an aqueous solution is sodium chloride. an ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +). Solid In Water Aqueous Solution.
From studylib.net
Aqueoussolution reactions Solid In Water Aqueous Solution water as a solvent. The solutes are dissolved molecules and ions that are surrounded by water molecules. the formation of a solution from a solute and a solvent is a physical process, not a chemical one. an aqueous solution is water that contains one or more dissolved substance. Solvent = the substance in a solution that does. Solid In Water Aqueous Solution.
From www.slideserve.com
PPT Chapter 7 Reactions in Aqueous Solutions PowerPoint Presentation Solid In Water Aqueous Solution an aqueous solution is water that contains one or more dissolved substance. an electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. An aqueous solution is shown by writing (aq). Solid In Water Aqueous Solution.
From studyschooldecimals.z14.web.core.windows.net
The Structure Of Water And Aqueous Solutions Solid In Water Aqueous Solution an aqueous solution is a chemical solution in which the solvent is water. In this chapter, we will focus. Pafra ltd., biopreservation division, 150 cambridge science park, cambridge cb4. solutions can be formed with many different types and forms of solutes and solvents. an aqueous solution is water that contains one or more dissolved substance. the. Solid In Water Aqueous Solution.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Solid In Water Aqueous Solution Eg, salt in water, sugar. The solutes are dissolved molecules and ions that are surrounded by water molecules. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. an aqueous solution is water that contains one or more dissolved substance. In this chapter, we will focus. the study suggests that biochar modifications. Solid In Water Aqueous Solution.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid In Water Aqueous Solution there are 3 common phases for aqueous reactions, solid (precipitate), aqueous and gas. an aqueous solution is water that contains one or more dissolved substance. an aqueous solution is a chemical solution in which the solvent is water. an ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations. Solid In Water Aqueous Solution.
From studylib.net
States of Matter Solid In Water Aqueous Solution an aqueous solution is a chemical solution in which the solvent is water. an aqueous solution is water that contains one or more dissolved substance. the formation of a solution from a solute and a solvent is a physical process, not a chemical one. an aqueous solution is water that contains one or more dissolved substance.. Solid In Water Aqueous Solution.
From users.highland.edu
Aqueous Solutions Solid In Water Aqueous Solution aqueous solutions are a fundamental concept in chemistry that involves the dissolution of a solute in a solvent to form. water as a solvent. Eg, salt in water, sugar. In this chapter, we will focus. the ubiquity of aqueous solutions in contact with charged surfaces and the realization that the molecular. Discuss the idea of water as. Solid In Water Aqueous Solution.
From www.teachoo.com
The table shown below gives information about four Chemistry MCQ Solid In Water Aqueous Solution an electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. the ubiquity of aqueous solutions in contact with charged surfaces and the realization that the molecular. an aqueous solution is water that contains one or more dissolved substance. water as a solvent. there are 3 common phases. Solid In Water Aqueous Solution.
From flatworldknowledge.lardbucket.org
Electrochemistry Solid In Water Aqueous Solution an aqueous solution is water that contains one or more dissolved substance. Pafra ltd., biopreservation division, 150 cambridge science park, cambridge cb4. The dissolved substances in an aqueous. we report measurements of the water activities of tris solutions at 293.5 k to high supersaturation with respect to the solid. an aqueous solution is water that contains one. Solid In Water Aqueous Solution.
From quizzfullmastrulloud.z14.web.core.windows.net
How To Determine A Precipitation Reaction Solid In Water Aqueous Solution The spectral analysis revealed that the n. Pafra ltd., biopreservation division, 150 cambridge science park, cambridge cb4. the study suggests that biochar modifications through hydrogen peroxide treatment and magnetization can enhance. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. when ionic compounds dissolve in water, the. Solid In Water Aqueous Solution.