Unstable Bromine Isotopes . The concept of isotopes is one of the most vital one in the field of chemistry. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. Isotopes of an element are atoms with the same atomic number but different mass numbers; The nucleus of a radioactive isotope is unstable;. Some elements have no stable isotopes and eventually decay to other elements. Isotopes of an element, therefore,. In this article, we shall know which are the stable and unstable. The two following tables list. Some naturally occurring and artificially produced isotopes are radioactive.
from www.chegg.com
Isotopes of an element are atoms with the same atomic number but different mass numbers; The concept of isotopes is one of the most vital one in the field of chemistry. Some elements have no stable isotopes and eventually decay to other elements. The nucleus of a radioactive isotope is unstable;. The two following tables list. In this article, we shall know which are the stable and unstable. Isotopes of an element, therefore,. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. Some naturally occurring and artificially produced isotopes are radioactive.
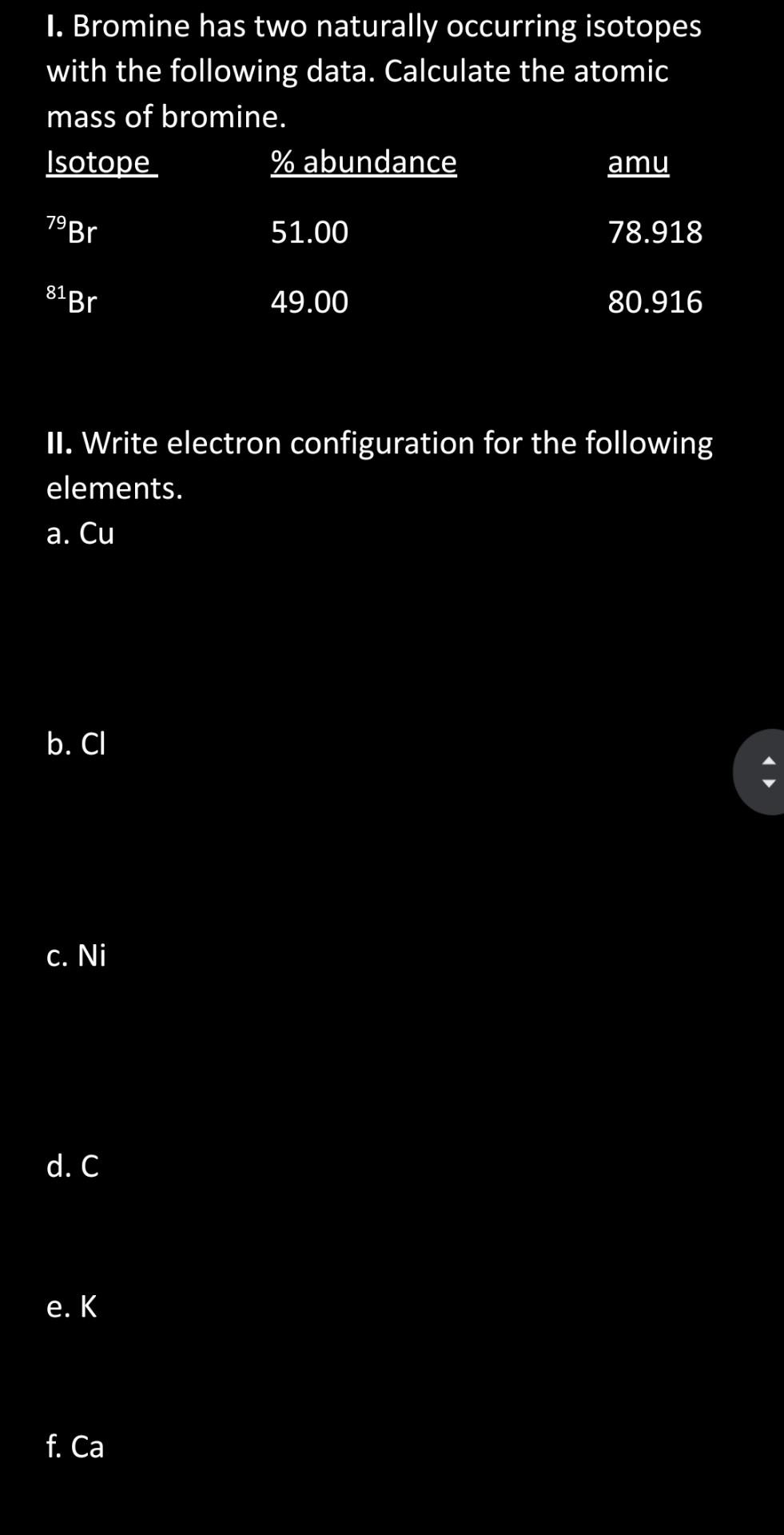
Solved I. Bromine has two naturally occurring isotopes with
Unstable Bromine Isotopes Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. Isotopes of an element are atoms with the same atomic number but different mass numbers; In this article, we shall know which are the stable and unstable. Isotopes of an element, therefore,. The two following tables list. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. The concept of isotopes is one of the most vital one in the field of chemistry. Some elements have no stable isotopes and eventually decay to other elements. Some naturally occurring and artificially produced isotopes are radioactive. The nucleus of a radioactive isotope is unstable;.
From www.youtube.com
Notation for Isotopes of Bromine (Br) YouTube Unstable Bromine Isotopes Isotopes of an element, therefore,. The concept of isotopes is one of the most vital one in the field of chemistry. Isotopes of an element are atoms with the same atomic number but different mass numbers; The two following tables list. In this article, we shall know which are the stable and unstable. Although most of the known elements have. Unstable Bromine Isotopes.
From www.pinterest.com
Unraveling the Mystery of Unstable Isotopes Unstable Bromine Isotopes The two following tables list. The nucleus of a radioactive isotope is unstable;. The concept of isotopes is one of the most vital one in the field of chemistry. In this article, we shall know which are the stable and unstable. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements. Unstable Bromine Isotopes.
From www.chegg.com
Solved I. Bromine has two naturally occurring isotopes with Unstable Bromine Isotopes The nucleus of a radioactive isotope is unstable;. Isotopes of an element are atoms with the same atomic number but different mass numbers; The concept of isotopes is one of the most vital one in the field of chemistry. The two following tables list. Isotopes of an element, therefore,. Although most of the known elements have at least one isotope. Unstable Bromine Isotopes.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Unstable Bromine Isotopes Isotopes of an element, therefore,. Isotopes of an element are atoms with the same atomic number but different mass numbers; Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. In this article, we shall know. Unstable Bromine Isotopes.
From material-properties.org
Bromine Periodic Table and Atomic Properties Unstable Bromine Isotopes The nucleus of a radioactive isotope is unstable;. The two following tables list. Isotopes of an element are atoms with the same atomic number but different mass numbers; The concept of isotopes is one of the most vital one in the field of chemistry. In this article, we shall know which are the stable and unstable. Some naturally occurring and. Unstable Bromine Isotopes.
From pediaa.com
Difference Between Stable and Unstable Isotopes Definition Unstable Bromine Isotopes The two following tables list. Some naturally occurring and artificially produced isotopes are radioactive. The nucleus of a radioactive isotope is unstable;. The concept of isotopes is one of the most vital one in the field of chemistry. In this article, we shall know which are the stable and unstable. Although most of the known elements have at least one. Unstable Bromine Isotopes.
From periodictable.com
Isotope data for bromine79 in the Periodic Table Unstable Bromine Isotopes Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. The two following tables list. Isotopes of an element are atoms with the same atomic number but different mass numbers; The concept of isotopes is one. Unstable Bromine Isotopes.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Unstable Bromine Isotopes The two following tables list. The concept of isotopes is one of the most vital one in the field of chemistry. Isotopes of an element, therefore,. Some naturally occurring and artificially produced isotopes are radioactive. The nucleus of a radioactive isotope is unstable;. Isotopes of an element are atoms with the same atomic number but different mass numbers; In this. Unstable Bromine Isotopes.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation ID2894903 Unstable Bromine Isotopes Isotopes of an element are atoms with the same atomic number but different mass numbers; Isotopes of an element, therefore,. Some naturally occurring and artificially produced isotopes are radioactive. The nucleus of a radioactive isotope is unstable;. The concept of isotopes is one of the most vital one in the field of chemistry. The two following tables list. Some elements. Unstable Bromine Isotopes.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear Unstable Bromine Isotopes Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. The concept of isotopes is one of the most vital one in the field of chemistry. In this article, we shall know which are the stable. Unstable Bromine Isotopes.
From www.slideshare.net
Atomic concepts Unstable Bromine Isotopes Isotopes of an element, therefore,. Some elements have no stable isotopes and eventually decay to other elements. Some naturally occurring and artificially produced isotopes are radioactive. The two following tables list. Isotopes of an element are atoms with the same atomic number but different mass numbers; The nucleus of a radioactive isotope is unstable;. The concept of isotopes is one. Unstable Bromine Isotopes.
From www.worksheetsplanet.com
What is an Isotope Definition of Isotope Unstable Bromine Isotopes Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. Isotopes of an element, therefore,. The two following tables list. The nucleus of a radioactive isotope is unstable;. In this article, we shall know which are. Unstable Bromine Isotopes.
From www.numerade.com
SOLVEDBromine is a diatomic molecule, and it has two isotopes, ^79 Br Unstable Bromine Isotopes The nucleus of a radioactive isotope is unstable;. Some elements have no stable isotopes and eventually decay to other elements. The two following tables list. Some naturally occurring and artificially produced isotopes are radioactive. Isotopes of an element, therefore,. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes. Unstable Bromine Isotopes.
From www.slideserve.com
PPT Bromine PowerPoint Presentation ID2319111 Unstable Bromine Isotopes Isotopes of an element, therefore,. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. The concept of isotopes is one of the most vital one in the field of chemistry. Isotopes of an element are. Unstable Bromine Isotopes.
From www.coursehero.com
[Solved] PQ14. Bromine has two naturally occurring isotopes. The most Unstable Bromine Isotopes In this article, we shall know which are the stable and unstable. The two following tables list. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. The concept of isotopes is one of the most. Unstable Bromine Isotopes.
From mavink.com
Lewis Dot Diagram For Bromine Unstable Bromine Isotopes In this article, we shall know which are the stable and unstable. The nucleus of a radioactive isotope is unstable;. The two following tables list. Isotopes of an element are atoms with the same atomic number but different mass numbers; Some elements have no stable isotopes and eventually decay to other elements. Isotopes of an element, therefore,. Although most of. Unstable Bromine Isotopes.
From www.istockphoto.com
Bromine Isotopes Stock Illustration Download Image Now Isotope Unstable Bromine Isotopes Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. Isotopes of an element are atoms with the same atomic number but different mass numbers; The concept of isotopes is one of the most vital one. Unstable Bromine Isotopes.
From martlabpro.com
What Is The Difference Between A Stable And Unstable Isotope? MartLabPro Unstable Bromine Isotopes The nucleus of a radioactive isotope is unstable;. Some elements have no stable isotopes and eventually decay to other elements. The two following tables list. Isotopes of an element are atoms with the same atomic number but different mass numbers; The concept of isotopes is one of the most vital one in the field of chemistry. Some naturally occurring and. Unstable Bromine Isotopes.
From large.stanford.edu
Unstable Isotopes Unstable Bromine Isotopes Some elements have no stable isotopes and eventually decay to other elements. The nucleus of a radioactive isotope is unstable;. Isotopes of an element, therefore,. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. Some. Unstable Bromine Isotopes.
From www.centraldatacore.com
035 Bromine Br Unstable Bromine Isotopes The nucleus of a radioactive isotope is unstable;. Isotopes of an element, therefore,. Isotopes of an element are atoms with the same atomic number but different mass numbers; The two following tables list. Some naturally occurring and artificially produced isotopes are radioactive. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all. Unstable Bromine Isotopes.
From www.slideserve.com
PPT Atomic Symbols and Isotopes PowerPoint Presentation, free Unstable Bromine Isotopes The nucleus of a radioactive isotope is unstable;. Some elements have no stable isotopes and eventually decay to other elements. In this article, we shall know which are the stable and unstable. Isotopes of an element, therefore,. The concept of isotopes is one of the most vital one in the field of chemistry. The two following tables list. Some naturally. Unstable Bromine Isotopes.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron Unstable Bromine Isotopes The concept of isotopes is one of the most vital one in the field of chemistry. Some elements have no stable isotopes and eventually decay to other elements. The nucleus of a radioactive isotope is unstable;. In this article, we shall know which are the stable and unstable. The two following tables list. Isotopes of an element are atoms with. Unstable Bromine Isotopes.
From www.researchgate.net
Patterns of the molecular ion with multiple chlorine or bromine atoms Unstable Bromine Isotopes Some naturally occurring and artificially produced isotopes are radioactive. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. The concept of isotopes is one of the most vital one in the field of chemistry. The. Unstable Bromine Isotopes.
From www.expii.com
Isotopes — Definition & Overview Expii Unstable Bromine Isotopes Some elements have no stable isotopes and eventually decay to other elements. In this article, we shall know which are the stable and unstable. The concept of isotopes is one of the most vital one in the field of chemistry. The two following tables list. The nucleus of a radioactive isotope is unstable;. Isotopes of an element, therefore,. Some naturally. Unstable Bromine Isotopes.
From www.mdpi.com
Minerals Free FullText Bromine Isotope Variations in Magmatic and Unstable Bromine Isotopes Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. The two following tables list. Isotopes of an element are atoms with the same atomic number but different mass numbers; The concept of isotopes is one. Unstable Bromine Isotopes.
From www.numerade.com
SOLVEDWrite a nuclear equation for the type of decay each of these Unstable Bromine Isotopes The concept of isotopes is one of the most vital one in the field of chemistry. In this article, we shall know which are the stable and unstable. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by. Unstable Bromine Isotopes.
From slideplayer.com
Herriman High Chemistry ppt download Unstable Bromine Isotopes Isotopes of an element, therefore,. Some elements have no stable isotopes and eventually decay to other elements. In this article, we shall know which are the stable and unstable. Isotopes of an element are atoms with the same atomic number but different mass numbers; The nucleus of a radioactive isotope is unstable;. Although most of the known elements have at. Unstable Bromine Isotopes.
From pediaa.com
Difference Between Stable and Unstable Isotopes Definition Unstable Bromine Isotopes The two following tables list. Isotopes of an element, therefore,. Some elements have no stable isotopes and eventually decay to other elements. The concept of isotopes is one of the most vital one in the field of chemistry. The nucleus of a radioactive isotope is unstable;. Although most of the known elements have at least one isotope whose atomic nucleus. Unstable Bromine Isotopes.
From www.numerade.com
SOLVEDNaturally occurring bromine consists of two isotopes, ^79 Br and Unstable Bromine Isotopes Some elements have no stable isotopes and eventually decay to other elements. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. In this article, we shall know which are the stable and unstable. The nucleus. Unstable Bromine Isotopes.
From martlabpro.com
What Is The Difference Between A Stable And Unstable Isotope? MartLabPro Unstable Bromine Isotopes The two following tables list. The nucleus of a radioactive isotope is unstable;. Some elements have no stable isotopes and eventually decay to other elements. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. Isotopes. Unstable Bromine Isotopes.
From www.chegg.com
Solved 8 Bromine (Br) has two isotopes, with masses of Unstable Bromine Isotopes The concept of isotopes is one of the most vital one in the field of chemistry. Isotopes of an element are atoms with the same atomic number but different mass numbers; The two following tables list. The nucleus of a radioactive isotope is unstable;. Some naturally occurring and artificially produced isotopes are radioactive. Although most of the known elements have. Unstable Bromine Isotopes.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Unstable Bromine Isotopes Isotopes of an element, therefore,. Some elements have no stable isotopes and eventually decay to other elements. Isotopes of an element are atoms with the same atomic number but different mass numbers; Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay,. Unstable Bromine Isotopes.
From www.isotopes.gov
New domestic supply chain Bromine76 and Bromine77 NIDC National Unstable Bromine Isotopes The nucleus of a radioactive isotope is unstable;. Some naturally occurring and artificially produced isotopes are radioactive. Isotopes of an element, therefore,. Isotopes of an element are atoms with the same atomic number but different mass numbers; The two following tables list. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all. Unstable Bromine Isotopes.
From www.youtube.com
Introduction to The Study of Unstable Isotopes Isotope Geology YouTube Unstable Bromine Isotopes In this article, we shall know which are the stable and unstable. Some naturally occurring and artificially produced isotopes are radioactive. The two following tables list. The nucleus of a radioactive isotope is unstable;. Some elements have no stable isotopes and eventually decay to other elements. Although most of the known elements have at least one isotope whose atomic nucleus. Unstable Bromine Isotopes.
From www.researchgate.net
Hydrogen, oxygen and bromine isotopic compositions for the water Unstable Bromine Isotopes The nucleus of a radioactive isotope is unstable;. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. Isotopes of an element are atoms with the same atomic number but different mass numbers; The concept of. Unstable Bromine Isotopes.