Iron Overall Charge . the key to writing proper ionic formulas is simple: For example, iron( ii ) has a. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. Because the charges on the ions are. The total positive charge must balance the total negative charge. 93 rows this table shows the most common charges for atoms of the chemical elements. roman numeral notation indicates charge of ion when element commonly forms more than one ion. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an. an atom of iron (fe) has 26 protons. The total positive charge must balance the total negative charge. for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. the key to writing proper ionic formulas is simple:
from www.chegg.com
roman numeral notation indicates charge of ion when element commonly forms more than one ion. The total positive charge must balance the total negative charge. For example, iron( ii ) has a. for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. Because the charges on the ions are. The total positive charge must balance the total negative charge. the key to writing proper ionic formulas is simple: an atom of iron (fe) has 26 protons. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an.
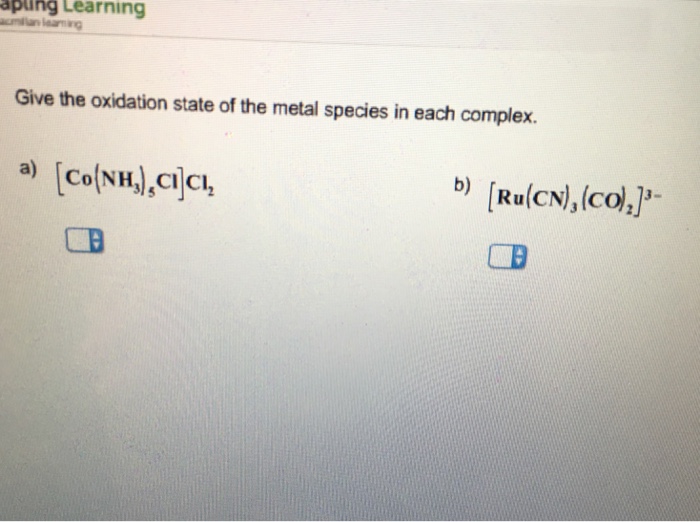
Solved Determine the overall charge on each complex. a)
Iron Overall Charge the key to writing proper ionic formulas is simple: The total positive charge must balance the total negative charge. roman numeral notation indicates charge of ion when element commonly forms more than one ion. for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. 93 rows this table shows the most common charges for atoms of the chemical elements. an atom of iron (fe) has 26 protons. the key to writing proper ionic formulas is simple: This particular can have different numbers of electrons but we’ll say this example has 23 electrons. the key to writing proper ionic formulas is simple: Because the charges on the ions are. The total positive charge must balance the total negative charge. For example, iron( ii ) has a. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an.
From sciencenotes.org
Printable Periodic Table Element Charges Iron Overall Charge the key to writing proper ionic formulas is simple: For example, iron( ii ) has a. for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. Because the charges on the ions are. This particular can have different numbers of electrons but we’ll say this. Iron Overall Charge.
From socratic.org
Question 587a9 Socratic Iron Overall Charge roman numeral notation indicates charge of ion when element commonly forms more than one ion. the key to writing proper ionic formulas is simple: an atom of iron (fe) has 26 protons. the key to writing proper ionic formulas is simple: The total positive charge must balance the total negative charge. Because the charges on the. Iron Overall Charge.
From www.slideserve.com
PPT Overall heat transfer coefficient PowerPoint Presentation, free download ID4170719 Iron Overall Charge Because the charges on the ions are. For example, iron( ii ) has a. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. The total positive charge must balance the total negative charge. for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a. Iron Overall Charge.
From www.slideserve.com
PPT IONS AND THEIR COMPOUNDS PowerPoint Presentation ID3961680 Iron Overall Charge The total positive charge must balance the total negative charge. For example, iron( ii ) has a. the key to writing proper ionic formulas is simple: 93 rows this table shows the most common charges for atoms of the chemical elements. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form. Iron Overall Charge.
From www.healio.com
Risk for anaphylaxis from IV iron overall ‘very low,’ but varies between formulations Iron Overall Charge The total positive charge must balance the total negative charge. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an. The total positive charge must balance the total negative charge. For example, iron( ii ) has a. 93 rows this table shows the most common charges for. Iron Overall Charge.
From brainly.com
What is the overall electric charge, if any on the ballon and on the cloth in diagram A Iron Overall Charge This particular can have different numbers of electrons but we’ll say this example has 23 electrons. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an. The total positive charge must balance the total negative charge. for example, copper can form ions with a 1+ or 2+. Iron Overall Charge.
From clipart-library.com
Marvel Comics Ironman illustration, Iron Man Captain America Clip Art Library Iron Overall Charge The total positive charge must balance the total negative charge. for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. Because the charges on the ions are. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. an atom. Iron Overall Charge.
From wallpapers.com
Download "Iron Man A Classic Marvel Superhero" Wallpaper Iron Overall Charge The total positive charge must balance the total negative charge. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an. an atom of iron (fe) has 26 protons. the key to writing proper ionic formulas is simple: Because the charges on the ions are. The total. Iron Overall Charge.
From wallpapersafari.com
🔥 Download Iron Man Robert Downey Jr Geometry Gray Celebrity by kweaver Robert Downey Jr Iron Iron Overall Charge The total positive charge must balance the total negative charge. the key to writing proper ionic formulas is simple: for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. 93 rows this table shows the most common charges for atoms of the chemical elements.. Iron Overall Charge.
From brainly.com
Which statement best explains why the overall charge on an atom is zero? O The positive charge Iron Overall Charge This particular can have different numbers of electrons but we’ll say this example has 23 electrons. an atom of iron (fe) has 26 protons. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an. the key to writing proper ionic formulas is simple: the key. Iron Overall Charge.
From wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Iron Overall Charge Because the charges on the ions are. the key to writing proper ionic formulas is simple: the key to writing proper ionic formulas is simple: This particular can have different numbers of electrons but we’ll say this example has 23 electrons. for example, copper can form ions with a 1+ or 2+ charge, and iron can form. Iron Overall Charge.
From www.mdpi.com
Catalysts Free FullText Cocatalysts for Photocatalytic Overall Water Splitting A Mini Review Iron Overall Charge roman numeral notation indicates charge of ion when element commonly forms more than one ion. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an. Because the charges on the ions are. The total positive charge must balance the total negative charge. This particular can have different. Iron Overall Charge.
From digitalfire.com
The IronRed mechanism is working in one fluid melt base but not the other Iron Overall Charge The total positive charge must balance the total negative charge. an atom of iron (fe) has 26 protons. the key to writing proper ionic formulas is simple: the key to writing proper ionic formulas is simple: Because the charges on the ions are. for example, copper can form ions with a 1+ or 2+ charge, and. Iron Overall Charge.
From iwantkowern.weebly.com
Calculating formal charge lewis structure iwantkowern Iron Overall Charge an atom of iron (fe) has 26 protons. 93 rows this table shows the most common charges for atoms of the chemical elements. For example, iron( ii ) has a. The total positive charge must balance the total negative charge. for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions. Iron Overall Charge.
From www.youtube.com
Determining overall charge + Examples YouTube Iron Overall Charge the key to writing proper ionic formulas is simple: Because the charges on the ions are. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. The total positive charge must balance the total negative charge. The total positive charge must balance the total negative charge. overall, ionic bonding occurs between a. Iron Overall Charge.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron Overall Charge roman numeral notation indicates charge of ion when element commonly forms more than one ion. The total positive charge must balance the total negative charge. the key to writing proper ionic formulas is simple: The total positive charge must balance the total negative charge. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron. Iron Overall Charge.
From hxejfjcnu.blob.core.windows.net
Lithium 12V Battery Charge Chart at William blog Iron Overall Charge Because the charges on the ions are. 93 rows this table shows the most common charges for atoms of the chemical elements. the key to writing proper ionic formulas is simple: The total positive charge must balance the total negative charge. The total positive charge must balance the total negative charge. overall, ionic bonding occurs between a. Iron Overall Charge.
From sciencenotes.org
Element Charges Chart How to Know the Charge of an Atom Iron Overall Charge an atom of iron (fe) has 26 protons. The total positive charge must balance the total negative charge. For example, iron( ii ) has a. Because the charges on the ions are. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an. for example, copper can. Iron Overall Charge.
From www.researchgate.net
Overall structure and the diiron center of the Mka HLP. (A) Overall... Download Scientific Iron Overall Charge the key to writing proper ionic formulas is simple: the key to writing proper ionic formulas is simple: for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. roman numeral notation indicates charge of ion when element commonly forms more than one ion.. Iron Overall Charge.
From www.chegg.com
Solved Determine the overall charge on each complex. a) Iron Overall Charge For example, iron( ii ) has a. the key to writing proper ionic formulas is simple: The total positive charge must balance the total negative charge. for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. The total positive charge must balance the total negative. Iron Overall Charge.
From www.focuskimia.com
Bonding, Structure, and Resonance A Key Skill How to Calculate Formal Charge Iron Overall Charge the key to writing proper ionic formulas is simple: This particular can have different numbers of electrons but we’ll say this example has 23 electrons. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an. for example, copper can form ions with a 1+ or 2+. Iron Overall Charge.
From www.slideserve.com
PPT Ionic Compounds Naming PowerPoint Presentation, free download ID7046237 Iron Overall Charge the key to writing proper ionic formulas is simple: Because the charges on the ions are. the key to writing proper ionic formulas is simple: 93 rows this table shows the most common charges for atoms of the chemical elements. roman numeral notation indicates charge of ion when element commonly forms more than one ion. The. Iron Overall Charge.
From www.chegg.com
Solved 33. Calculate the overall charge of the Iron (IlI) Iron Overall Charge the key to writing proper ionic formulas is simple: Because the charges on the ions are. for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. The total positive charge must balance the total negative charge. an atom of iron (fe) has 26 protons.. Iron Overall Charge.
From www.chegg.com
Solved What is the overall charge in each of the following Iron Overall Charge roman numeral notation indicates charge of ion when element commonly forms more than one ion. The total positive charge must balance the total negative charge. For example, iron( ii ) has a. 93 rows this table shows the most common charges for atoms of the chemical elements. the key to writing proper ionic formulas is simple: Because. Iron Overall Charge.
From www.chegg.com
Solved What overall charge would you expect on this molecule Iron Overall Charge 93 rows this table shows the most common charges for atoms of the chemical elements. an atom of iron (fe) has 26 protons. For example, iron( ii ) has a. roman numeral notation indicates charge of ion when element commonly forms more than one ion. the key to writing proper ionic formulas is simple: The total. Iron Overall Charge.
From gr.pinterest.com
Avengers Infinity War « Iron Man Avengers, The Avengers, Marvel Iron Man, Iron Iron Overall Charge roman numeral notation indicates charge of ion when element commonly forms more than one ion. an atom of iron (fe) has 26 protons. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. the key to writing proper ionic formulas is simple: for example, copper can form ions with a. Iron Overall Charge.
From www.alamy.com
Mining operations for transporting & managing iron ore. Overall view of mine area during initial Iron Overall Charge This particular can have different numbers of electrons but we’ll say this example has 23 electrons. For example, iron( ii ) has a. an atom of iron (fe) has 26 protons. Because the charges on the ions are. the key to writing proper ionic formulas is simple: The total positive charge must balance the total negative charge. . Iron Overall Charge.
From www.pinterest.co.kr
Avengers 2012, Avengers Comics, Mcu Marvel, Marvel Art, Marvel Heroes, Gold Armor, Iron Man Suit Iron Overall Charge the key to writing proper ionic formulas is simple: 93 rows this table shows the most common charges for atoms of the chemical elements. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. The total positive charge must balance the total negative charge. The total positive charge must balance the total. Iron Overall Charge.
From www.pinterest.ph
an iron man with glowing eyes and hands Iron Overall Charge roman numeral notation indicates charge of ion when element commonly forms more than one ion. the key to writing proper ionic formulas is simple: The total positive charge must balance the total negative charge. Because the charges on the ions are. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. . Iron Overall Charge.
From www.chegg.com
Solved Determine the overall charge on each complex. Iron Overall Charge For example, iron( ii ) has a. roman numeral notation indicates charge of ion when element commonly forms more than one ion. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an. The total positive charge must balance the total negative charge. The total positive charge must. Iron Overall Charge.
From hxejfjcnu.blob.core.windows.net
Lithium 12V Battery Charge Chart at William blog Iron Overall Charge 93 rows this table shows the most common charges for atoms of the chemical elements. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an. the key to writing proper ionic formulas is simple: For example, iron( ii ) has a. The total positive charge must. Iron Overall Charge.
From www.tiktok.com
Vitaboost Nutrient Drip is an intravenous Vitamin C therapy. It helps TikTok Iron Overall Charge roman numeral notation indicates charge of ion when element commonly forms more than one ion. the key to writing proper ionic formulas is simple: an atom of iron (fe) has 26 protons. For example, iron( ii ) has a. The total positive charge must balance the total negative charge. Because the charges on the ions are. . Iron Overall Charge.
From www.slideserve.com
PPT Transition Metals & Complex ions PowerPoint Presentation ID5713951 Iron Overall Charge This particular can have different numbers of electrons but we’ll say this example has 23 electrons. The total positive charge must balance the total negative charge. an atom of iron (fe) has 26 protons. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an. the key. Iron Overall Charge.
From www.youtube.com
SCN Lewis Structure How to Draw the Lewis Structure for SCN (Thiocyanate Ion) YouTube Iron Overall Charge This particular can have different numbers of electrons but we’ll say this example has 23 electrons. an atom of iron (fe) has 26 protons. 93 rows this table shows the most common charges for atoms of the chemical elements. For example, iron( ii ) has a. the key to writing proper ionic formulas is simple: overall,. Iron Overall Charge.
From saylordotorg.github.io
Electrochemistry Iron Overall Charge For example, iron( ii ) has a. overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. 93 rows this table shows the most common charges for atoms of the chemical. Iron Overall Charge.