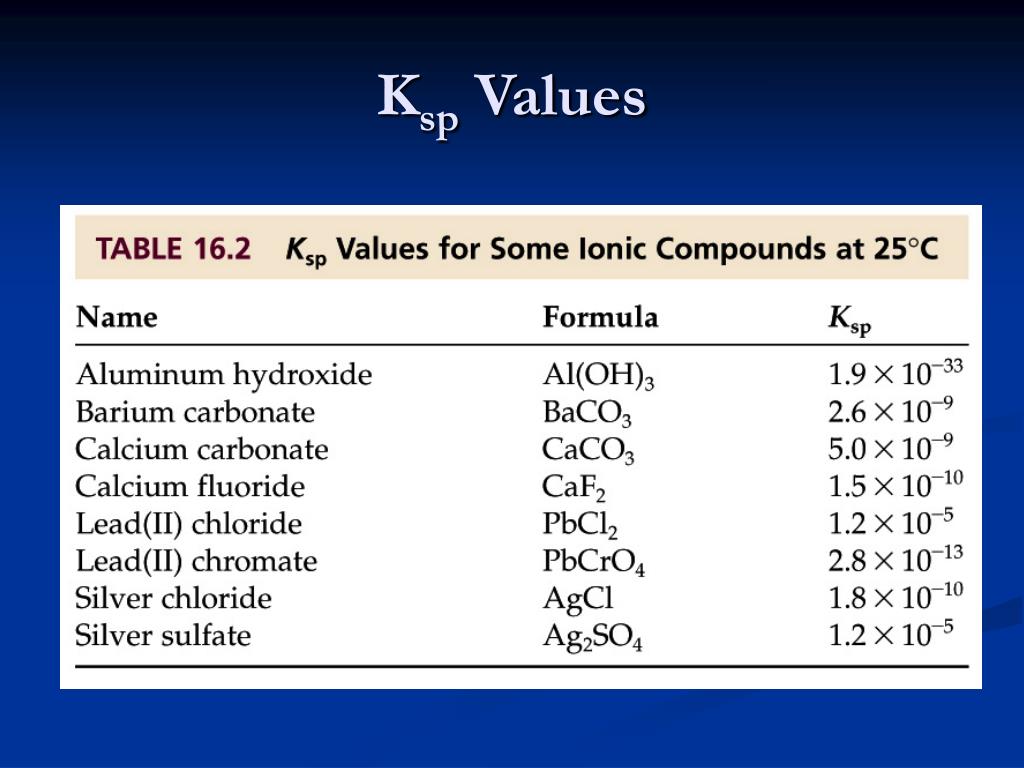
Table Salt Ksp . The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (k sp) of the salt. Below are the values of the ksp product constant for the most common salts. The ksp values for some common salts at 25 ˚c are listed in the table below. We hope they will prove usefull to you. If there are any other salts. Because the concentration of a pure solid such as ca 3. The k sp values are indicators of the solubility of. 176 rows the value of the solubility constant depends only on temperature for a given salt. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of the salt.
from www.slideserve.com
Because the concentration of a pure solid such as ca 3. Below are the values of the ksp product constant for the most common salts. The ksp values for some common salts at 25 ˚c are listed in the table below. The k sp values are indicators of the solubility of. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of the salt. If there are any other salts. The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (k sp) of the salt. 176 rows the value of the solubility constant depends only on temperature for a given salt. We hope they will prove usefull to you.
PPT Solubility PowerPoint Presentation, free download ID6260427
Table Salt Ksp Because the concentration of a pure solid such as ca 3. The k sp values are indicators of the solubility of. We hope they will prove usefull to you. If there are any other salts. The ksp values for some common salts at 25 ˚c are listed in the table below. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of the salt. The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (k sp) of the salt. Because the concentration of a pure solid such as ca 3. 176 rows the value of the solubility constant depends only on temperature for a given salt. Below are the values of the ksp product constant for the most common salts.
From www.slideserve.com
PPT Creating Compounds PowerPoint Presentation, free download ID Table Salt Ksp 176 rows the value of the solubility constant depends only on temperature for a given salt. Below are the values of the ksp product constant for the most common salts. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t. Table Salt Ksp.
From www.thoughtco.com
Chemical Composition of Table Salt Table Salt Ksp Because the concentration of a pure solid such as ca 3. The k sp values are indicators of the solubility of. The ksp values for some common salts at 25 ˚c are listed in the table below. Below are the values of the ksp product constant for the most common salts. The equilibrium constant for the dissolution of a sparingly. Table Salt Ksp.
From www.numerade.com
SOLVED Arrange the salts by their molar solubility in water. Consult Table Salt Ksp If there are any other salts. The k sp values are indicators of the solubility of. The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (k sp) of the salt. The ksp values for some common salts at 25 ˚c are listed in the table below. The equilibrium constant for the dissolution of a. Table Salt Ksp.
From www.numerade.com
SOLVED Arrange the salts by their molar solubility in water Consult Table Salt Ksp The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of the salt. Below are the values of the ksp product constant for the most common salts. The k sp values are indicators of the solubility of. The. Table Salt Ksp.
From www.youtube.com
Calculating the Ksp of an insoluble salt YouTube Table Salt Ksp 176 rows the value of the solubility constant depends only on temperature for a given salt. The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (k sp) of the salt. If there are any other salts. Below are the values of the ksp product constant for the most common salts. Because the concentration of. Table Salt Ksp.
From www.numerade.com
Arrange the salts by their molar solubility in water Consult the table Table Salt Ksp The k sp values are indicators of the solubility of. If there are any other salts. The ksp values for some common salts at 25 ˚c are listed in the table below. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u. Table Salt Ksp.
From www.chegg.com
Solved Experiment 8 The Solubility Product Constant (Ksp) Table Salt Ksp The ksp values for some common salts at 25 ˚c are listed in the table below. 176 rows the value of the solubility constant depends only on temperature for a given salt. The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (k sp) of the salt. We hope they will prove usefull to you.. Table Salt Ksp.
From www.numerade.com
SOLVEDThe salts in Table 151, with the possible exception of the Table Salt Ksp The ksp values for some common salts at 25 ˚c are listed in the table below. Because the concentration of a pure solid such as ca 3. Below are the values of the ksp product constant for the most common salts. 176 rows the value of the solubility constant depends only on temperature for a given salt. We hope they. Table Salt Ksp.
From www.youtube.com
Lecture 7 Definition Ksp solubility product YouTube Table Salt Ksp The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of the salt. The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (k sp) of the salt. If there are any other. Table Salt Ksp.
From www.slideserve.com
PPT Ksp and the Common Ion Effect PowerPoint Presentation, free Table Salt Ksp The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (k sp) of the salt. Below are the values of the ksp product constant for the most common salts. If there are any other salts. We hope they will prove usefull to you. The ksp values for some common salts at 25 ˚c are listed. Table Salt Ksp.
From www.numerade.com
Experiment 8 The Solubility Product Constant (Ksp) for a Sparingly Table Salt Ksp The ksp values for some common salts at 25 ˚c are listed in the table below. Because the concentration of a pure solid such as ca 3. Below are the values of the ksp product constant for the most common salts. The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (k sp) of the. Table Salt Ksp.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID6260427 Table Salt Ksp Because the concentration of a pure solid such as ca 3. The ksp values for some common salts at 25 ˚c are listed in the table below. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of. Table Salt Ksp.
From www.slideserve.com
PPT The Solubility Product Constant, K sp PowerPoint Presentation Table Salt Ksp We hope they will prove usefull to you. 176 rows the value of the solubility constant depends only on temperature for a given salt. The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (k sp) of the salt. The k sp values are indicators of the solubility of. Below are the values of the. Table Salt Ksp.
From www.slideserve.com
PPT Salts and Solubility PowerPoint Presentation, free download ID Table Salt Ksp Below are the values of the ksp product constant for the most common salts. The ksp values for some common salts at 25 ˚c are listed in the table below. The k sp values are indicators of the solubility of. If there are any other salts. 176 rows the value of the solubility constant depends only on temperature for a. Table Salt Ksp.
From www.studocu.com
Salts General Chemistry Ksp and Solubility Calculations of a Salt Table Salt Ksp The ksp values for some common salts at 25 ˚c are listed in the table below. We hope they will prove usefull to you. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of the salt. 176. Table Salt Ksp.
From z-cm.blogspot.com
Ksp Table Decoration Examples Table Salt Ksp The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (k sp) of the salt. The k sp values are indicators of the solubility of. The ksp values for some common salts at 25 ˚c are listed in the table below. Because the concentration of a pure solid such as ca 3. We hope they. Table Salt Ksp.
From www.chegg.com
Solved Calculate Ksp for the following salts (AgSCN, Table Salt Ksp If there are any other salts. The k sp values are indicators of the solubility of. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of the salt. 176 rows the value of the solubility constant depends. Table Salt Ksp.
From www.chegg.com
Solved Given the following Ksp chart, rank the salts listed Table Salt Ksp The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (k sp) of the salt. We hope they will prove usefull to you. The ksp values for some common salts at 25 ˚c are listed in the table below. 176 rows the value of the solubility constant depends only on temperature for a given salt.. Table Salt Ksp.
From www.chegg.com
Solved Determination of Ksp of a Tartrate Salt Lab Table Salt Ksp 176 rows the value of the solubility constant depends only on temperature for a given salt. The ksp values for some common salts at 25 ˚c are listed in the table below. The k sp values are indicators of the solubility of. Below are the values of the ksp product constant for the most common salts. Because the concentration of. Table Salt Ksp.
From www.chegg.com
Solved calcualte molar solubility of the salt, Ksp of the Table Salt Ksp The k sp values are indicators of the solubility of. The ksp values for some common salts at 25 ˚c are listed in the table below. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of the. Table Salt Ksp.
From askfilo.com
al formula Table 7.9 The Solubility Product Constants, equilibrium Ksp Table Salt Ksp The ksp values for some common salts at 25 ˚c are listed in the table below. 176 rows the value of the solubility constant depends only on temperature for a given salt. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u. Table Salt Ksp.
From www.thoughtco.com
Chemical Composition of Table Salt Table Salt Ksp We hope they will prove usefull to you. The ksp values for some common salts at 25 ˚c are listed in the table below. 176 rows the value of the solubility constant depends only on temperature for a given salt. The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (k sp) of the salt.. Table Salt Ksp.
From www.youtube.com
The values of Ksp of two sparingly soluble salts Ni(OH)2 and AgCN are 2 Table Salt Ksp The ksp values for some common salts at 25 ˚c are listed in the table below. We hope they will prove usefull to you. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of the salt. The. Table Salt Ksp.
From www.numerade.com
SOLVEDThe salts in Table 8.5, with the possible exception of the Table Salt Ksp The k sp values are indicators of the solubility of. The ksp values for some common salts at 25 ˚c are listed in the table below. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of the. Table Salt Ksp.
From www.numerade.com
SOLVED Rank the salts given in the table below from least soluble to Table Salt Ksp We hope they will prove usefull to you. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of the salt. Below are the values of the ksp product constant for the most common salts. The ksp values. Table Salt Ksp.
From slidetodoc.com
Salts and Solubility Activity 3 Solution Equilibrium and Table Salt Ksp If there are any other salts. Because the concentration of a pure solid such as ca 3. 176 rows the value of the solubility constant depends only on temperature for a given salt. The k sp values are indicators of the solubility of. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u. Table Salt Ksp.
From www.sliderbase.com
Solubility Equilibria Presentation Chemistry Table Salt Ksp Because the concentration of a pure solid such as ca 3. The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (k sp) of the salt. If there are any other salts. 176 rows the value of the solubility constant depends only on temperature for a given salt. We hope they will prove usefull to. Table Salt Ksp.
From www.numerade.com
Arrange the salts by their molar solubility in water Consult the table Table Salt Ksp We hope they will prove usefull to you. If there are any other salts. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of the salt. 176 rows the value of the solubility constant depends only on. Table Salt Ksp.
From courses.lumenlearning.com
Solubility Equilibria Boundless Chemistry Table Salt Ksp 176 rows the value of the solubility constant depends only on temperature for a given salt. The ksp values for some common salts at 25 ˚c are listed in the table below. If there are any other salts. We hope they will prove usefull to you. Because the concentration of a pure solid such as ca 3. Below are the. Table Salt Ksp.
From www.numerade.com
SOLVEDThe salts in Table 16.1, with the possible exception of the Table Salt Ksp The ksp values for some common salts at 25 ˚c are listed in the table below. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of the salt. Because the concentration of a pure solid such as. Table Salt Ksp.
From www.youtube.com
Ksp Molar Solubility, Ice Tables, & Common Ion Effect YouTube Table Salt Ksp The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (k sp) of the salt. 176 rows the value of the solubility constant depends only on temperature for a given salt. Because the concentration of a pure solid such as ca 3. If there are any other salts. We hope they will prove usefull to. Table Salt Ksp.
From www.bartleby.com
Answered Arrange the salts by their molar… bartleby Table Salt Ksp The k sp values are indicators of the solubility of. 176 rows the value of the solubility constant depends only on temperature for a given salt. Below are the values of the ksp product constant for the most common salts. We hope they will prove usefull to you. If there are any other salts. The equilibrium constant for the dissolution. Table Salt Ksp.
From www.numerade.com
SOLVED Write the answer clearly Q. Solubility equilibria In the table Table Salt Ksp The k sp values are indicators of the solubility of. 176 rows the value of the solubility constant depends only on temperature for a given salt. The ksp values for some common salts at 25 ˚c are listed in the table below. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b. Table Salt Ksp.
From www.numerade.com
SOLVED Write the solubility equilibrium equation and solubility Table Salt Ksp We hope they will prove usefull to you. 176 rows the value of the solubility constant depends only on temperature for a given salt. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of the salt. The. Table Salt Ksp.
From www.scribd.com
KSP chart Hydroxide Cobalt Table Salt Ksp If there are any other salts. The ksp values for some common salts at 25 ˚c are listed in the table below. The equilibrium constant for the dissolution of a sparingly soluble salt is the s o l u b i l i t y p r o d u c t (k ) of the salt. 176 rows the. Table Salt Ksp.