How To Write Electron Configuration For Magnesium . Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². The shorthand electron configuration (or noble. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². electron configuration chart of all elements is mentioned in the table below. Today we will tell you about the electron configuration of the mg. to write the orbital diagram for the magnesium atom (mg) first we need to. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. magnesium electron configuration. Magnesium has two valence electrons in. The ninth most abundant element in the universe. how do you write magnesium with electron configuration?
from schematicellsmounty.z21.web.core.windows.net
The ninth most abundant element in the universe. magnesium electron configuration. Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Today we will tell you about the electron configuration of the mg. to write the orbital diagram for the magnesium atom (mg) first we need to. The shorthand electron configuration (or noble. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². electron configuration chart of all elements is mentioned in the table below. Magnesium has two valence electrons in.
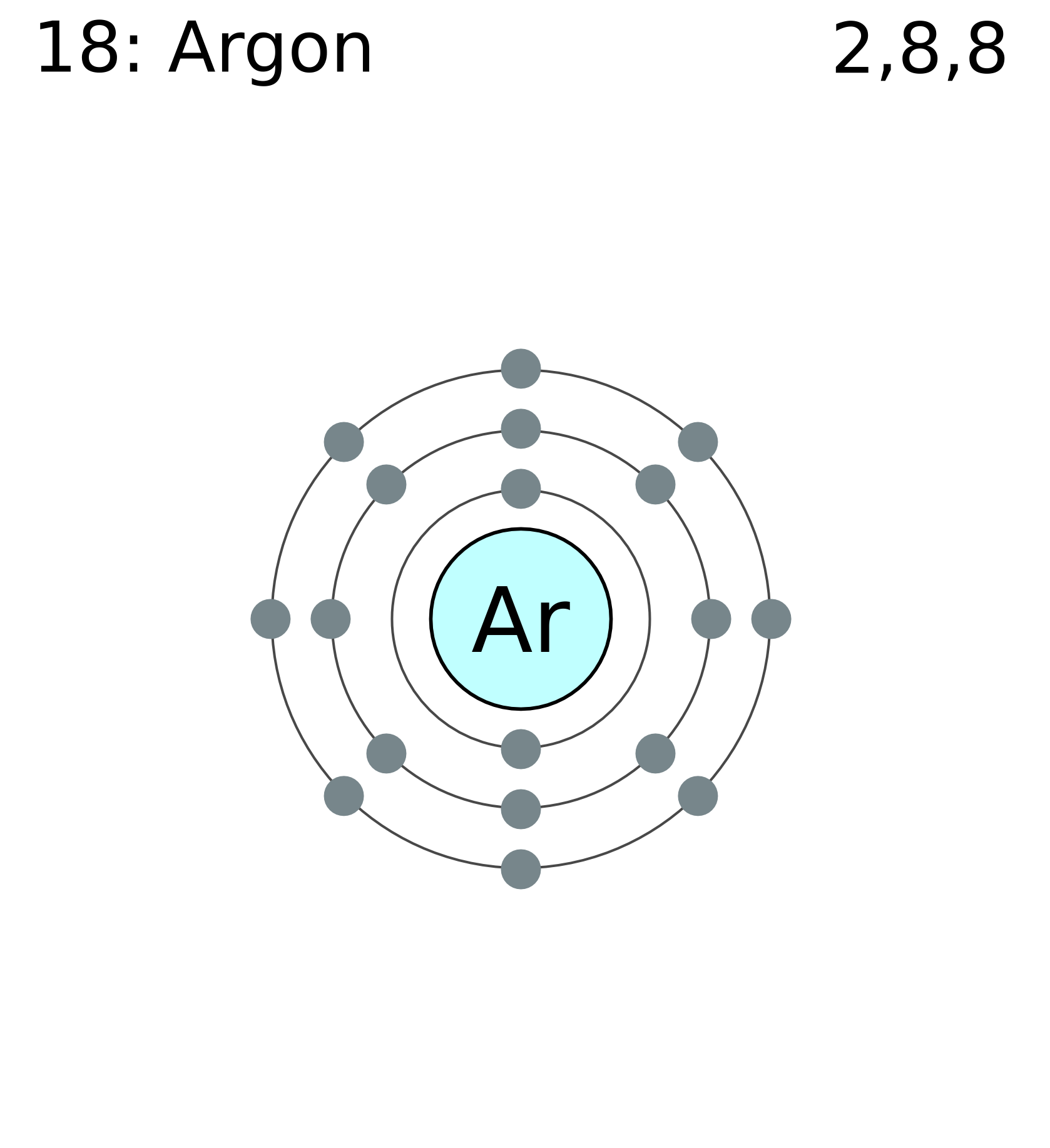
Atomic Orbital Energy Diagram For Argon
How To Write Electron Configuration For Magnesium magnesium electron configuration. Today we will tell you about the electron configuration of the mg. electron configuration chart of all elements is mentioned in the table below. The ninth most abundant element in the universe. magnesium electron configuration. The shorthand electron configuration (or noble. how do you write magnesium with electron configuration? Magnesium has two valence electrons in. to write the orbital diagram for the magnesium atom (mg) first we need to. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².
From schematicfixspellers.z22.web.core.windows.net
Aluminum Atom Diagram How To Write Electron Configuration For Magnesium Magnesium has two valence electrons in. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble. magnesium electron configuration. how do you write magnesium with electron. How To Write Electron Configuration For Magnesium.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image How To Write Electron Configuration For Magnesium Magnesium has two valence electrons in. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. how do you write magnesium with electron configuration? electron configuration chart of all elements is mentioned in the table below. The ninth most abundant element in the universe. Write. How To Write Electron Configuration For Magnesium.
From www.toppr.com
Write the electron dot structure of magnesium and oxygen. How To Write Electron Configuration For Magnesium The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². to write the orbital diagram for the magnesium atom (mg) first we need to. Magnesium has two valence electrons in. Today we will tell you about the electron configuration of the mg. The shorthand electron configuration (or noble. The ninth most abundant element in the universe. electron configuration. How To Write Electron Configuration For Magnesium.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline How To Write Electron Configuration For Magnesium Magnesium has two valence electrons in. electron configuration chart of all elements is mentioned in the table below. how do you write magnesium with electron configuration? to write the orbital diagram for the magnesium atom (mg) first we need to. The shorthand electron configuration (or noble. Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². Today we. How To Write Electron Configuration For Magnesium.
From learningschoolhanerydd2l.z21.web.core.windows.net
How To Write Electron Configuration Diagrams How To Write Electron Configuration For Magnesium electron configuration chart of all elements is mentioned in the table below. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². magnesium electron configuration. to write the orbital diagram for the magnesium atom (mg) first we need to. The shorthand electron configuration (or noble. All elements of group 2 have the same configuration of an electron. How To Write Electron Configuration For Magnesium.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram How To Write Electron Configuration For Magnesium The shorthand electron configuration (or noble. The ninth most abundant element in the universe. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Today we will tell you about the electron configuration of the mg. how do you write magnesium with electron configuration? Write magnesium’s. How To Write Electron Configuration For Magnesium.
From www.thesciencehive.co.uk
Atomic Structure and Electron Configuration (AQA) — the science hive How To Write Electron Configuration For Magnesium Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Today we will tell you about the electron configuration of the mg. The ninth most abundant element in the universe. electron. How To Write Electron Configuration For Magnesium.
From ellisrestroulner94.blogspot.com
Silicon Electron Configuration How Many Unpaired Electrons Ellis How To Write Electron Configuration For Magnesium The shorthand electron configuration (or noble. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². electron configuration chart of all elements is mentioned in the table below. how do you write magnesium with electron configuration? Magnesium has two valence electrons in. Today we will tell you about the. How To Write Electron Configuration For Magnesium.
From www.animalia-life.club
Magnesium Electron Configuration How To Write Electron Configuration For Magnesium Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The ninth most abundant element in the universe. to write the orbital diagram for the magnesium atom (mg) first we need. How To Write Electron Configuration For Magnesium.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium How To Write Electron Configuration For Magnesium The ninth most abundant element in the universe. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². electron configuration chart of all elements is mentioned in the table below. Magnesium has two valence electrons in. Today we will tell you about the electron configuration of the mg. how do you write magnesium with electron configuration? The shorthand. How To Write Electron Configuration For Magnesium.
From www.mikrora.com
File Electron Configuration Magnesium Svg Best Diagram Collection How To Write Electron Configuration For Magnesium Today we will tell you about the electron configuration of the mg. to write the orbital diagram for the magnesium atom (mg) first we need to. The ninth most abundant element in the universe. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Magnesium has. How To Write Electron Configuration For Magnesium.
From www.youtube.com
Electronic configuration for Magnesium (Mg) spdf Trick Chemistry How To Write Electron Configuration For Magnesium to write the orbital diagram for the magnesium atom (mg) first we need to. electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble. how do you write magnesium with electron configuration? The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Today we will tell you about the. How To Write Electron Configuration For Magnesium.
From manualdatasiphonogam.z21.web.core.windows.net
Diagram Of Ion How To Write Electron Configuration For Magnesium The ninth most abundant element in the universe. Magnesium has two valence electrons in. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. magnesium electron configuration. how do you write magnesium with electron configuration? electron configuration chart of all elements is mentioned in. How To Write Electron Configuration For Magnesium.
From www.coursehero.com
[Solved] Write the short form electron configuration for magnesium How To Write Electron Configuration For Magnesium Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. how do you write magnesium with electron configuration? Magnesium has two valence electrons in. The ninth most abundant element in the universe. The electron configuration of magnesium. How To Write Electron Configuration For Magnesium.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube How To Write Electron Configuration For Magnesium to write the orbital diagram for the magnesium atom (mg) first we need to. Magnesium has two valence electrons in. Today we will tell you about the electron configuration of the mg. magnesium electron configuration. The ninth most abundant element in the universe. All elements of group 2 have the same configuration of an electron in the outer. How To Write Electron Configuration For Magnesium.
From www.newtondesk.com
magnesium electron configuration Newton Desk How To Write Electron Configuration For Magnesium to write the orbital diagram for the magnesium atom (mg) first we need to. Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². The shorthand electron configuration (or noble. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². how do you write magnesium with electron configuration? Today we will tell you about the electron configuration of the. How To Write Electron Configuration For Magnesium.
From libloytelestichs.z21.web.core.windows.net
Electron Configurations And Orbital Diagrams Worksheets How To Write Electron Configuration For Magnesium The ninth most abundant element in the universe. magnesium electron configuration. how do you write magnesium with electron configuration? Magnesium has two valence electrons in. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Today we will tell you about the electron configuration of. How To Write Electron Configuration For Magnesium.
From www.pinterest.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo How To Write Electron Configuration For Magnesium The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². electron configuration chart of all elements is mentioned in the table below. Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². how do you write magnesium with electron configuration? The shorthand electron configuration (or noble. Today we will tell you about the electron configuration of the mg. The. How To Write Electron Configuration For Magnesium.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements How To Write Electron Configuration For Magnesium to write the orbital diagram for the magnesium atom (mg) first we need to. The shorthand electron configuration (or noble. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². how do you write magnesium with electron configuration? Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². The ninth most abundant element in the universe. Today we will. How To Write Electron Configuration For Magnesium.
From lessoncampushotelman.z5.web.core.windows.net
How To Draw The Electron Configuration How To Write Electron Configuration For Magnesium magnesium electron configuration. electron configuration chart of all elements is mentioned in the table below. Magnesium has two valence electrons in. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The ninth most abundant element in the universe. The shorthand electron configuration (or noble.. How To Write Electron Configuration For Magnesium.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for How To Write Electron Configuration For Magnesium The ninth most abundant element in the universe. Magnesium has two valence electrons in. Today we will tell you about the electron configuration of the mg. The shorthand electron configuration (or noble. to write the orbital diagram for the magnesium atom (mg) first we need to. electron configuration chart of all elements is mentioned in the table below.. How To Write Electron Configuration For Magnesium.
From schematicellsmounty.z21.web.core.windows.net
Atomic Orbital Energy Diagram For Argon How To Write Electron Configuration For Magnesium The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². magnesium electron configuration. The shorthand electron configuration (or noble. electron configuration chart of all elements is mentioned in the table below. Today we will tell you about the electron configuration of the mg. All elements of group 2 have the same configuration of an electron in the outer. How To Write Electron Configuration For Magnesium.
From circuitwiringshiite77.z21.web.core.windows.net
Box Diagram Of Electron Configuration How To Write Electron Configuration For Magnesium magnesium electron configuration. to write the orbital diagram for the magnesium atom (mg) first we need to. how do you write magnesium with electron configuration? Today we will tell you about the electron configuration of the mg. The shorthand electron configuration (or noble. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Write magnesium’s electron configuration. How To Write Electron Configuration For Magnesium.
From manualdatasiphonogam.z21.web.core.windows.net
Dot And Cross Diagram Of Chlorine How To Write Electron Configuration For Magnesium The shorthand electron configuration (or noble. magnesium electron configuration. how do you write magnesium with electron configuration? All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The ninth most abundant element in the universe. electron configuration chart of all elements is mentioned in. How To Write Electron Configuration For Magnesium.
From ecurrencythailand.com
What Is The Number Of Electrons In Mg And Mg2+ Ion? Top 10 Best Answers How To Write Electron Configuration For Magnesium The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Magnesium has two valence electrons in. The shorthand electron configuration (or noble. The ninth most abundant element in the universe. to write the orbital diagram for. How To Write Electron Configuration For Magnesium.
From celbncbc.blob.core.windows.net
Magnesium Ion Number Of Electrons at Joan Harbert blog How To Write Electron Configuration For Magnesium to write the orbital diagram for the magnesium atom (mg) first we need to. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Today we will tell you about the electron configuration of the mg. how do you write magnesium with electron configuration? Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². The ninth most abundant element. How To Write Electron Configuration For Magnesium.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram How To Write Electron Configuration For Magnesium Today we will tell you about the electron configuration of the mg. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in. The shorthand electron configuration (or noble. magnesium electron configuration. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal. How To Write Electron Configuration For Magnesium.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram How To Write Electron Configuration For Magnesium electron configuration chart of all elements is mentioned in the table below. Magnesium has two valence electrons in. Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². The shorthand electron configuration (or noble. The ninth most abundant element in the universe. to write the orbital diagram for the magnesium atom (mg) first we need to. The electron configuration. How To Write Electron Configuration For Magnesium.
From schematicellsmounty.z21.web.core.windows.net
Atomic Orbital Energy Diagram For Argon How To Write Electron Configuration For Magnesium magnesium electron configuration. to write the orbital diagram for the magnesium atom (mg) first we need to. Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². electron configuration chart of all elements is mentioned in the table below. The ninth most abundant element in the universe. Today we will tell you about the electron configuration of the. How To Write Electron Configuration For Magnesium.
From www.animalia-life.club
Magnesium Electron Configuration How To Write Electron Configuration For Magnesium Today we will tell you about the electron configuration of the mg. how do you write magnesium with electron configuration? All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The shorthand electron configuration (or noble. Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². Magnesium. How To Write Electron Configuration For Magnesium.
From cetvcjtg.blob.core.windows.net
Magnesium Electron Cloud at Edward Tobler blog How To Write Electron Configuration For Magnesium to write the orbital diagram for the magnesium atom (mg) first we need to. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The shorthand electron configuration (or noble. Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². magnesium electron configuration. Magnesium has two valence electrons in. All elements of group 2 have the same configuration of. How To Write Electron Configuration For Magnesium.
From loekclpfw.blob.core.windows.net
Electron Configuration For Ca 2 at Jennifer Abbott blog How To Write Electron Configuration For Magnesium The ninth most abundant element in the universe. to write the orbital diagram for the magnesium atom (mg) first we need to. electron configuration chart of all elements is mentioned in the table below. how do you write magnesium with electron configuration? The shorthand electron configuration (or noble. Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s².. How To Write Electron Configuration For Magnesium.
From brainly.in
Diagramatic sketch of electronic configuration of magnesium (atomic How To Write Electron Configuration For Magnesium Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². The ninth most abundant element in the universe. The shorthand electron configuration (or noble. electron configuration chart of all elements is mentioned in the table below. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. . How To Write Electron Configuration For Magnesium.
From valenceelectrons.com
Electron Configuration for Magnesium(Mg, Mg2+ ion) How To Write Electron Configuration For Magnesium All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. magnesium electron configuration. Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². to write the orbital diagram for the magnesium atom (mg) first we need to. The ninth most abundant element in the universe. . How To Write Electron Configuration For Magnesium.
From cetvcjtg.blob.core.windows.net
Magnesium Electron Cloud at Edward Tobler blog How To Write Electron Configuration For Magnesium magnesium electron configuration. The ninth most abundant element in the universe. Write magnesium’s electron configuration as 1s² 2s² 2p⁶ 3s². to write the orbital diagram for the magnesium atom (mg) first we need to. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. . How To Write Electron Configuration For Magnesium.