Single Use Medical Devices Fda . produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. this page provides information to help health care facilities understand the use of reprocessed medical devices. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for.
from www.meddevicecorp.com
reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. this page provides information to help health care facilities understand the use of reprocessed medical devices.
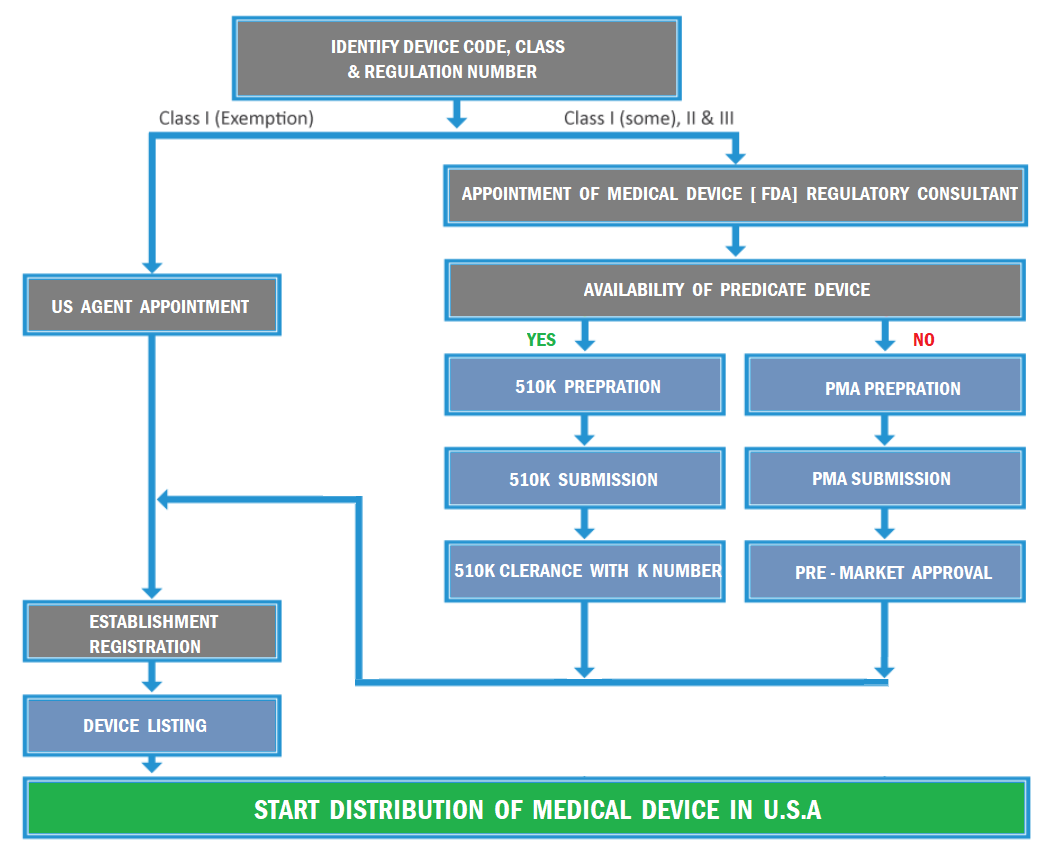
US FDA Registration Service of Food, Drugs, Medical Devices
Single Use Medical Devices Fda reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. this page provides information to help health care facilities understand the use of reprocessed medical devices.
From es.slideshare.net
Regulation of Medical Devices in US Single Use Medical Devices Fda within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. this page provides information to help health care facilities understand the use of reprocessed medical devices. reusable medical devices are. Single Use Medical Devices Fda.
From www.vrogue.co
Fda Launches New page To Promote Use Of Symbols In vrogue.co Single Use Medical Devices Fda produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. this page provides. Single Use Medical Devices Fda.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Single Use Medical Devices Fda within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. this page provides. Single Use Medical Devices Fda.
From www.vhv.rs
Fda Medical Device Symbols Advanced Practices In Nursing Journal, HD Single Use Medical Devices Fda produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. this page provides. Single Use Medical Devices Fda.
From www.alamy.com
Full vector set of sterilized and disinfectant symbols for medical Single Use Medical Devices Fda reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. this page provides. Single Use Medical Devices Fda.
From angelanjohnson.com
Medical Devices Angela N Johnson Single Use Medical Devices Fda this page provides information to help health care facilities understand the use of reprocessed medical devices. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. reusable medical devices are. Single Use Medical Devices Fda.
From www.mdpi.com
Sustainability Free FullText Green Servitization in the SingleUse Single Use Medical Devices Fda within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. this page provides information to help health care facilities understand the use of reprocessed medical devices. produce medical devices designated as. Single Use Medical Devices Fda.
From coremedsurgical.com
Can a Single Use Medical Device Be Used More Than Once? https Single Use Medical Devices Fda produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. this page provides information to help health care facilities understand the use of reprocessed medical devices. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. reusable medical devices are. Single Use Medical Devices Fda.
From medidee.com
[ARTICLE] Combination Products Similarities and Differences of EU and Single Use Medical Devices Fda produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. this page provides information to help health care facilities understand the use of reprocessed medical devices. within the department of health. Single Use Medical Devices Fda.
From www.dotcompliance.com
Quality Management System (QMS) for Medical Device Dot Compliance Single Use Medical Devices Fda reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. this page provides information to help health care facilities understand the use of reprocessed medical devices. produce medical devices designated as. Single Use Medical Devices Fda.
From actismedical.com
Single Use Devices (SUD) Actis Medical Single Use Medical Devices Fda produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. this page provides. Single Use Medical Devices Fda.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Single Use Medical Devices Fda produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. this page provides information to help health care facilities understand the use of reprocessed medical devices. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. within the department of health. Single Use Medical Devices Fda.
From exyuxzjps.blob.core.windows.net
Medical Device Regulations Fda at Lois Ogrady blog Single Use Medical Devices Fda this page provides information to help health care facilities understand the use of reprocessed medical devices. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. reusable medical devices are. Single Use Medical Devices Fda.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Single Use Medical Devices Fda within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. this page provides information to help health care facilities understand the use of reprocessed medical devices. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. reusable medical devices are. Single Use Medical Devices Fda.
From www.ibottles.net
Single Use Medical Device Innovative Bottles Single Use Medical Devices Fda this page provides information to help health care facilities understand the use of reprocessed medical devices. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. within the department of health. Single Use Medical Devices Fda.
From medtechintelligence.com
Column Compliance Date Approaching for FDA Unique Device Identifiers Single Use Medical Devices Fda this page provides information to help health care facilities understand the use of reprocessed medical devices. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. produce medical devices designated as. Single Use Medical Devices Fda.
From www.dreamstime.com
Single Use Medical Device Symbol Stock Illustrations 17 Single Use Single Use Medical Devices Fda reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. this page provides information to help health care facilities understand the use of reprocessed medical devices. within the department of health. Single Use Medical Devices Fda.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Single Use Medical Devices Fda within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. this page provides. Single Use Medical Devices Fda.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Single Use Medical Devices Fda within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. this page provides information to help health care facilities understand the use of reprocessed medical devices. produce medical devices designated as. Single Use Medical Devices Fda.
From www.vrogue.co
Fda Medical Device Labeling Requirements Presentation vrogue.co Single Use Medical Devices Fda within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. this page provides. Single Use Medical Devices Fda.
From www.hcinnovationgroup.com
UDI Not just for manufacturers anymore Healthcare Innovation Single Use Medical Devices Fda reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. this page provides information to help health care facilities understand the use of reprocessed medical devices. produce medical devices designated as. Single Use Medical Devices Fda.
From leanraqa.com
FDA Submission for Combination Products Regulatory + Quality Single Use Medical Devices Fda this page provides information to help health care facilities understand the use of reprocessed medical devices. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. reusable medical devices are. Single Use Medical Devices Fda.
From mavink.com
Medical Device Labeling Symbols Single Use Medical Devices Fda produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. this page provides information to help health care facilities understand the use of reprocessed medical devices. reusable medical devices are. Single Use Medical Devices Fda.
From hospitalsmagazine.com
Singleuse medical devices HOSPITALS MAGAZINE Single Use Medical Devices Fda produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. this page provides information to help health care facilities understand the use of reprocessed medical devices. within the department of health. Single Use Medical Devices Fda.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Single Use Medical Devices Fda within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. this page provides. Single Use Medical Devices Fda.
From onlinelibrary.wiley.com
Do Healthcare Professionals Comprehend Standardized Symbols Present on Single Use Medical Devices Fda this page provides information to help health care facilities understand the use of reprocessed medical devices. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. produce medical devices designated as. Single Use Medical Devices Fda.
From www.dreamstime.com
Singleuse Medical Devices Symbol. Concept of Packaging Stock Vector Single Use Medical Devices Fda within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. this page provides. Single Use Medical Devices Fda.
From www.mordorintelligence.com
AsiaPacific Singleuse Medical Device Reprocessing Market Size Single Use Medical Devices Fda produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. this page provides information to help health care facilities understand the use of reprocessed medical devices. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. within the department of health. Single Use Medical Devices Fda.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Single Use Medical Devices Fda within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. this page provides. Single Use Medical Devices Fda.
From www.youtube.com
Considerations for Third Party Reprocessing of SingleUse Medical Single Use Medical Devices Fda within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. this page provides. Single Use Medical Devices Fda.
From www.jointcommission.org
Symbol table for device labeling Single Use Medical Devices Fda this page provides information to help health care facilities understand the use of reprocessed medical devices. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. within the department of health. Single Use Medical Devices Fda.
From www.meddevicecorp.com
US FDA Registration Service of Food, Drugs, Medical Devices Single Use Medical Devices Fda reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. this page provides. Single Use Medical Devices Fda.
From www.arenasolutions.com
Class I Device Definition Arena Single Use Medical Devices Fda produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. this page provides information to help health care facilities understand the use of reprocessed medical devices. reusable medical devices are. Single Use Medical Devices Fda.
From www.medline.ca
Medical Device Reprocessing Medline Canada Single Use Medical Devices Fda reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. this page provides information to help health care facilities understand the use of reprocessed medical devices. produce medical devices designated as “single use,” due to demand by health care organizations, the complexity of newly. within the department of health. Single Use Medical Devices Fda.
From issuu.com
SingleUse Medical Device Reprocessing Market Industry Analysis, Size Single Use Medical Devices Fda reusable medical devices are devices that health care providers can reuse to diagnose and treat multiple patients. this page provides information to help health care facilities understand the use of reprocessed medical devices. within the department of health and human services (hhs), the food and drug administration (fda) is responsible for. produce medical devices designated as. Single Use Medical Devices Fda.