Copper Sulphate And Steel Wool Equation . Chapter 88.4 chemical reactions investigation 8.6 p 322 jacaranda science. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so 4. When steel wool is added to a copper sulfate solution, a displacement reaction occurs. An iron nail or steel wool is placed into a solution of cuso4 and copper is plated onto the iron surface. It forms hydrates cuso4·nh2o, where n can range from 1 to 7. The iron in the steel wool displaces the. The pentahydrate (n = 5), a bright. When solid nickel metal is put into an aqueous solution of copper (ii) sulfate, solid copper metal and a solution of nickel sulfate. The reverse (copper in an. We will show a little video of how they react with each other and how. In the presentation we will be mentioning the physical and chemical properties of copper (ii)sulfate and steel wool. If the steel wool is clean and free of soap, copper from the copper sulphate will deposit onto it and the steel wool will become copper.
from www.transtutors.com
We will show a little video of how they react with each other and how. Chapter 88.4 chemical reactions investigation 8.6 p 322 jacaranda science. An iron nail or steel wool is placed into a solution of cuso4 and copper is plated onto the iron surface. When steel wool is added to a copper sulfate solution, a displacement reaction occurs. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so 4. When solid nickel metal is put into an aqueous solution of copper (ii) sulfate, solid copper metal and a solution of nickel sulfate. It forms hydrates cuso4·nh2o, where n can range from 1 to 7. The iron in the steel wool displaces the. The reverse (copper in an. If the steel wool is clean and free of soap, copper from the copper sulphate will deposit onto it and the steel wool will become copper.
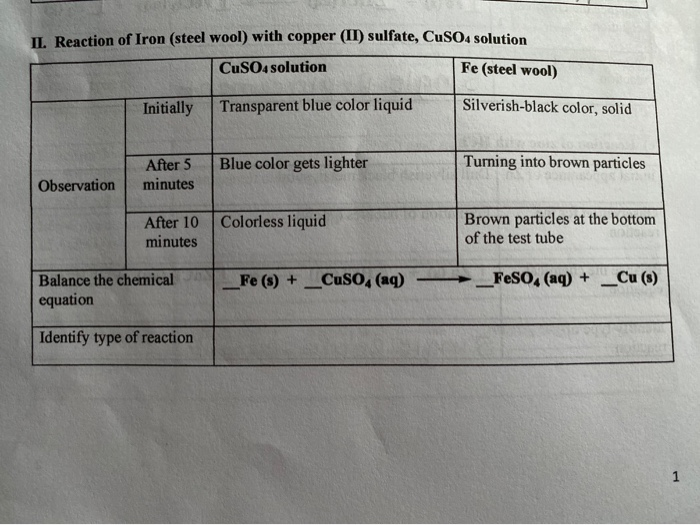
(Get Answer) II. Reaction of Iron (steel wool) with copper (II
Copper Sulphate And Steel Wool Equation When solid nickel metal is put into an aqueous solution of copper (ii) sulfate, solid copper metal and a solution of nickel sulfate. The pentahydrate (n = 5), a bright. It forms hydrates cuso4·nh2o, where n can range from 1 to 7. An iron nail or steel wool is placed into a solution of cuso4 and copper is plated onto the iron surface. In the presentation we will be mentioning the physical and chemical properties of copper (ii)sulfate and steel wool. If the steel wool is clean and free of soap, copper from the copper sulphate will deposit onto it and the steel wool will become copper. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so 4. Chapter 88.4 chemical reactions investigation 8.6 p 322 jacaranda science. The reverse (copper in an. When solid nickel metal is put into an aqueous solution of copper (ii) sulfate, solid copper metal and a solution of nickel sulfate. The iron in the steel wool displaces the. We will show a little video of how they react with each other and how. When steel wool is added to a copper sulfate solution, a displacement reaction occurs.
From amizaraspecialitychemicals.co.in
Understanding the Uses and Benefits of Copper Sulphate in Agriculture Copper Sulphate And Steel Wool Equation The iron in the steel wool displaces the. Chapter 88.4 chemical reactions investigation 8.6 p 322 jacaranda science. When solid nickel metal is put into an aqueous solution of copper (ii) sulfate, solid copper metal and a solution of nickel sulfate. If the steel wool is clean and free of soap, copper from the copper sulphate will deposit onto it. Copper Sulphate And Steel Wool Equation.
From brainly.in
What do you observe when iron nail are added to copper sulphate Copper Sulphate And Steel Wool Equation The iron in the steel wool displaces the. Chapter 88.4 chemical reactions investigation 8.6 p 322 jacaranda science. We will show a little video of how they react with each other and how. An iron nail or steel wool is placed into a solution of cuso4 and copper is plated onto the iron surface. Copper (ii) sulfate is an inorganic. Copper Sulphate And Steel Wool Equation.
From www.shimico.com
Copper Sulfate and the methods of production Shimico blog Copper Sulphate And Steel Wool Equation Chapter 88.4 chemical reactions investigation 8.6 p 322 jacaranda science. An iron nail or steel wool is placed into a solution of cuso4 and copper is plated onto the iron surface. The pentahydrate (n = 5), a bright. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so 4. The reverse (copper in an. When steel wool. Copper Sulphate And Steel Wool Equation.
From brainly.in
What are the products of Copper sulphate + sodium hydroxide? Brainly.in Copper Sulphate And Steel Wool Equation An iron nail or steel wool is placed into a solution of cuso4 and copper is plated onto the iron surface. The pentahydrate (n = 5), a bright. We will show a little video of how they react with each other and how. If the steel wool is clean and free of soap, copper from the copper sulphate will deposit. Copper Sulphate And Steel Wool Equation.
From www.youtube.com
Displacement reaction class 10 in hindi Displacement reaction of Copper Sulphate And Steel Wool Equation Copper (ii) sulfate is an inorganic compound with the chemical formula cu so 4. We will show a little video of how they react with each other and how. An iron nail or steel wool is placed into a solution of cuso4 and copper is plated onto the iron surface. In the presentation we will be mentioning the physical and. Copper Sulphate And Steel Wool Equation.
From www.animalia-life.club
Crystallisation Of Copper Sulphate Copper Sulphate And Steel Wool Equation Chapter 88.4 chemical reactions investigation 8.6 p 322 jacaranda science. It forms hydrates cuso4·nh2o, where n can range from 1 to 7. In the presentation we will be mentioning the physical and chemical properties of copper (ii)sulfate and steel wool. We will show a little video of how they react with each other and how. When steel wool is added. Copper Sulphate And Steel Wool Equation.
From www.slideserve.com
PPT Powerpoint to help with unit 22 PowerPoint Presentation, free Copper Sulphate And Steel Wool Equation We will show a little video of how they react with each other and how. Chapter 88.4 chemical reactions investigation 8.6 p 322 jacaranda science. The iron in the steel wool displaces the. The reverse (copper in an. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so 4. In the presentation we will be mentioning the. Copper Sulphate And Steel Wool Equation.
From sciencenotes.org
How to Make Copper Sulfate (Copper Sulphate) Copper Sulphate And Steel Wool Equation If the steel wool is clean and free of soap, copper from the copper sulphate will deposit onto it and the steel wool will become copper. In the presentation we will be mentioning the physical and chemical properties of copper (ii)sulfate and steel wool. We will show a little video of how they react with each other and how. When. Copper Sulphate And Steel Wool Equation.
From www.alamy.com
A tuft of steel wool has been submerged in a solution of copper sulfate Copper Sulphate And Steel Wool Equation In the presentation we will be mentioning the physical and chemical properties of copper (ii)sulfate and steel wool. The iron in the steel wool displaces the. Chapter 88.4 chemical reactions investigation 8.6 p 322 jacaranda science. An iron nail or steel wool is placed into a solution of cuso4 and copper is plated onto the iron surface. When steel wool. Copper Sulphate And Steel Wool Equation.
From www.numerade.com
SOLVED Solid copper reacts with solid sulfur(S8) to form solid copper Copper Sulphate And Steel Wool Equation When solid nickel metal is put into an aqueous solution of copper (ii) sulfate, solid copper metal and a solution of nickel sulfate. It forms hydrates cuso4·nh2o, where n can range from 1 to 7. We will show a little video of how they react with each other and how. The iron in the steel wool displaces the. Chapter 88.4. Copper Sulphate And Steel Wool Equation.
From brainly.in
Write the word equation to show what happens when Iron reacts with Copper Sulphate And Steel Wool Equation If the steel wool is clean and free of soap, copper from the copper sulphate will deposit onto it and the steel wool will become copper. In the presentation we will be mentioning the physical and chemical properties of copper (ii)sulfate and steel wool. The reverse (copper in an. The pentahydrate (n = 5), a bright. The iron in the. Copper Sulphate And Steel Wool Equation.
From www.teachoo.com
MCQ (Sample Paper) In reaction of iron with copper sulphate solution Copper Sulphate And Steel Wool Equation We will show a little video of how they react with each other and how. The reverse (copper in an. The iron in the steel wool displaces the. When steel wool is added to a copper sulfate solution, a displacement reaction occurs. In the presentation we will be mentioning the physical and chemical properties of copper (ii)sulfate and steel wool.. Copper Sulphate And Steel Wool Equation.
From www.youtube.com
Copper and Sulfur reaction YouTube Copper Sulphate And Steel Wool Equation Chapter 88.4 chemical reactions investigation 8.6 p 322 jacaranda science. It forms hydrates cuso4·nh2o, where n can range from 1 to 7. We will show a little video of how they react with each other and how. In the presentation we will be mentioning the physical and chemical properties of copper (ii)sulfate and steel wool. The reverse (copper in an.. Copper Sulphate And Steel Wool Equation.
From www.transtutors.com
(Get Answer) II. Reaction of Iron (steel wool) with copper (II Copper Sulphate And Steel Wool Equation When solid nickel metal is put into an aqueous solution of copper (ii) sulfate, solid copper metal and a solution of nickel sulfate. The iron in the steel wool displaces the. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so 4. It forms hydrates cuso4·nh2o, where n can range from 1 to 7. Chapter 88.4 chemical. Copper Sulphate And Steel Wool Equation.
From answerzonecelluloid.z21.web.core.windows.net
Steel Wool Chemical Formula Copper Sulphate And Steel Wool Equation In the presentation we will be mentioning the physical and chemical properties of copper (ii)sulfate and steel wool. It forms hydrates cuso4·nh2o, where n can range from 1 to 7. When solid nickel metal is put into an aqueous solution of copper (ii) sulfate, solid copper metal and a solution of nickel sulfate. Copper (ii) sulfate is an inorganic compound. Copper Sulphate And Steel Wool Equation.
From www.youtube.com
Electrolysis Of Copper(ii) Sulphate Using Copper Electrodes YouTube Copper Sulphate And Steel Wool Equation The pentahydrate (n = 5), a bright. If the steel wool is clean and free of soap, copper from the copper sulphate will deposit onto it and the steel wool will become copper. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so 4. In the presentation we will be mentioning the physical and chemical properties of. Copper Sulphate And Steel Wool Equation.
From www.youtube.com
Copper sulfate and steel wool YouTube Copper Sulphate And Steel Wool Equation When solid nickel metal is put into an aqueous solution of copper (ii) sulfate, solid copper metal and a solution of nickel sulfate. When steel wool is added to a copper sulfate solution, a displacement reaction occurs. If the steel wool is clean and free of soap, copper from the copper sulphate will deposit onto it and the steel wool. Copper Sulphate And Steel Wool Equation.
From www.goldhilleducation.co.uk
IGCSE Chemistry Mychem Copper Sulphate And Steel Wool Equation We will show a little video of how they react with each other and how. An iron nail or steel wool is placed into a solution of cuso4 and copper is plated onto the iron surface. The pentahydrate (n = 5), a bright. In the presentation we will be mentioning the physical and chemical properties of copper (ii)sulfate and steel. Copper Sulphate And Steel Wool Equation.
From www.youtube.com
Lab Activity Reaction of Copper Sulphate and Iron, Class 7 Physics Copper Sulphate And Steel Wool Equation We will show a little video of how they react with each other and how. If the steel wool is clean and free of soap, copper from the copper sulphate will deposit onto it and the steel wool will become copper. Chapter 88.4 chemical reactions investigation 8.6 p 322 jacaranda science. In the presentation we will be mentioning the physical. Copper Sulphate And Steel Wool Equation.
From www.slideshare.net
Replacement reactions Copper Sulphate And Steel Wool Equation When steel wool is added to a copper sulfate solution, a displacement reaction occurs. The reverse (copper in an. The iron in the steel wool displaces the. We will show a little video of how they react with each other and how. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so 4. The pentahydrate (n =. Copper Sulphate And Steel Wool Equation.
From www.chegg.com
Solved II. Reaction of Iron (steel wool) with copper (II) Copper Sulphate And Steel Wool Equation An iron nail or steel wool is placed into a solution of cuso4 and copper is plated onto the iron surface. When steel wool is added to a copper sulfate solution, a displacement reaction occurs. The iron in the steel wool displaces the. If the steel wool is clean and free of soap, copper from the copper sulphate will deposit. Copper Sulphate And Steel Wool Equation.
From scienceinfo.com
Copper Sulphate Preparation, Properties, health hazards Copper Sulphate And Steel Wool Equation An iron nail or steel wool is placed into a solution of cuso4 and copper is plated onto the iron surface. We will show a little video of how they react with each other and how. When solid nickel metal is put into an aqueous solution of copper (ii) sulfate, solid copper metal and a solution of nickel sulfate. The. Copper Sulphate And Steel Wool Equation.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Copper Sulphate And Steel Wool Equation An iron nail or steel wool is placed into a solution of cuso4 and copper is plated onto the iron surface. The pentahydrate (n = 5), a bright. In the presentation we will be mentioning the physical and chemical properties of copper (ii)sulfate and steel wool. The reverse (copper in an. When solid nickel metal is put into an aqueous. Copper Sulphate And Steel Wool Equation.
From blog.thepipingmart.com
Exploring the Different Reactions of Zinc to Acetic Acid and Copper Copper Sulphate And Steel Wool Equation An iron nail or steel wool is placed into a solution of cuso4 and copper is plated onto the iron surface. When solid nickel metal is put into an aqueous solution of copper (ii) sulfate, solid copper metal and a solution of nickel sulfate. In the presentation we will be mentioning the physical and chemical properties of copper (ii)sulfate and. Copper Sulphate And Steel Wool Equation.
From www.youtube.com
Displacement reaction (reaction of copper sulphate and iron) Explained Copper Sulphate And Steel Wool Equation The pentahydrate (n = 5), a bright. When solid nickel metal is put into an aqueous solution of copper (ii) sulfate, solid copper metal and a solution of nickel sulfate. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so 4. We will show a little video of how they react with each other and how. When. Copper Sulphate And Steel Wool Equation.
From byjus.com
Why does the color of copper sulphate change when an iron nail is kept Copper Sulphate And Steel Wool Equation Copper (ii) sulfate is an inorganic compound with the chemical formula cu so 4. The reverse (copper in an. If the steel wool is clean and free of soap, copper from the copper sulphate will deposit onto it and the steel wool will become copper. The pentahydrate (n = 5), a bright. The iron in the steel wool displaces the.. Copper Sulphate And Steel Wool Equation.
From nail.ftempo.com
Iron Nail Copper Ii Sulfate Word Equation Nail Ftempo Copper Sulphate And Steel Wool Equation Chapter 88.4 chemical reactions investigation 8.6 p 322 jacaranda science. It forms hydrates cuso4·nh2o, where n can range from 1 to 7. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so 4. The pentahydrate (n = 5), a bright. The reverse (copper in an. In the presentation we will be mentioning the physical and chemical properties. Copper Sulphate And Steel Wool Equation.
From www.youtube.com
steel wool and copper II sulphate YouTube Copper Sulphate And Steel Wool Equation The pentahydrate (n = 5), a bright. When steel wool is added to a copper sulfate solution, a displacement reaction occurs. It forms hydrates cuso4·nh2o, where n can range from 1 to 7. Chapter 88.4 chemical reactions investigation 8.6 p 322 jacaranda science. The reverse (copper in an. If the steel wool is clean and free of soap, copper from. Copper Sulphate And Steel Wool Equation.
From www.youtube.com
How to Write the Formula for Copper (II) sulfate YouTube Copper Sulphate And Steel Wool Equation The pentahydrate (n = 5), a bright. Chapter 88.4 chemical reactions investigation 8.6 p 322 jacaranda science. The iron in the steel wool displaces the. When steel wool is added to a copper sulfate solution, a displacement reaction occurs. When solid nickel metal is put into an aqueous solution of copper (ii) sulfate, solid copper metal and a solution of. Copper Sulphate And Steel Wool Equation.
From testbook.com
Copper Sulphate Formula Structure, Preparation, Properties & Uses Copper Sulphate And Steel Wool Equation The pentahydrate (n = 5), a bright. It forms hydrates cuso4·nh2o, where n can range from 1 to 7. The iron in the steel wool displaces the. The reverse (copper in an. Chapter 88.4 chemical reactions investigation 8.6 p 322 jacaranda science. When solid nickel metal is put into an aqueous solution of copper (ii) sulfate, solid copper metal and. Copper Sulphate And Steel Wool Equation.
From www.numerade.com
SOLVED reacting steel wool (iron) with copper (ii) sulfate what is Copper Sulphate And Steel Wool Equation We will show a little video of how they react with each other and how. The reverse (copper in an. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so 4. In the presentation we will be mentioning the physical and chemical properties of copper (ii)sulfate and steel wool. An iron nail or steel wool is placed. Copper Sulphate And Steel Wool Equation.
From www.youtube.com
Investigation 8 6 Steel Wool in Copper Sulfate YouTube Copper Sulphate And Steel Wool Equation Copper (ii) sulfate is an inorganic compound with the chemical formula cu so 4. The reverse (copper in an. The pentahydrate (n = 5), a bright. In the presentation we will be mentioning the physical and chemical properties of copper (ii)sulfate and steel wool. An iron nail or steel wool is placed into a solution of cuso4 and copper is. Copper Sulphate And Steel Wool Equation.
From www.youtube.com
Copper Sulfate and Sodium Hydroxide YouTube Copper Sulphate And Steel Wool Equation If the steel wool is clean and free of soap, copper from the copper sulphate will deposit onto it and the steel wool will become copper. It forms hydrates cuso4·nh2o, where n can range from 1 to 7. When solid nickel metal is put into an aqueous solution of copper (ii) sulfate, solid copper metal and a solution of nickel. Copper Sulphate And Steel Wool Equation.
From studylibraryintroit.z14.web.core.windows.net
Formula For Steel Wool Copper Sulphate And Steel Wool Equation The pentahydrate (n = 5), a bright. The reverse (copper in an. If the steel wool is clean and free of soap, copper from the copper sulphate will deposit onto it and the steel wool will become copper. When steel wool is added to a copper sulfate solution, a displacement reaction occurs. An iron nail or steel wool is placed. Copper Sulphate And Steel Wool Equation.
From www.youtube.com
Iron metal + Copper(II) sulfate solution reaction Timelapse 100x YouTube Copper Sulphate And Steel Wool Equation If the steel wool is clean and free of soap, copper from the copper sulphate will deposit onto it and the steel wool will become copper. The iron in the steel wool displaces the. In the presentation we will be mentioning the physical and chemical properties of copper (ii)sulfate and steel wool. The reverse (copper in an. Chapter 88.4 chemical. Copper Sulphate And Steel Wool Equation.