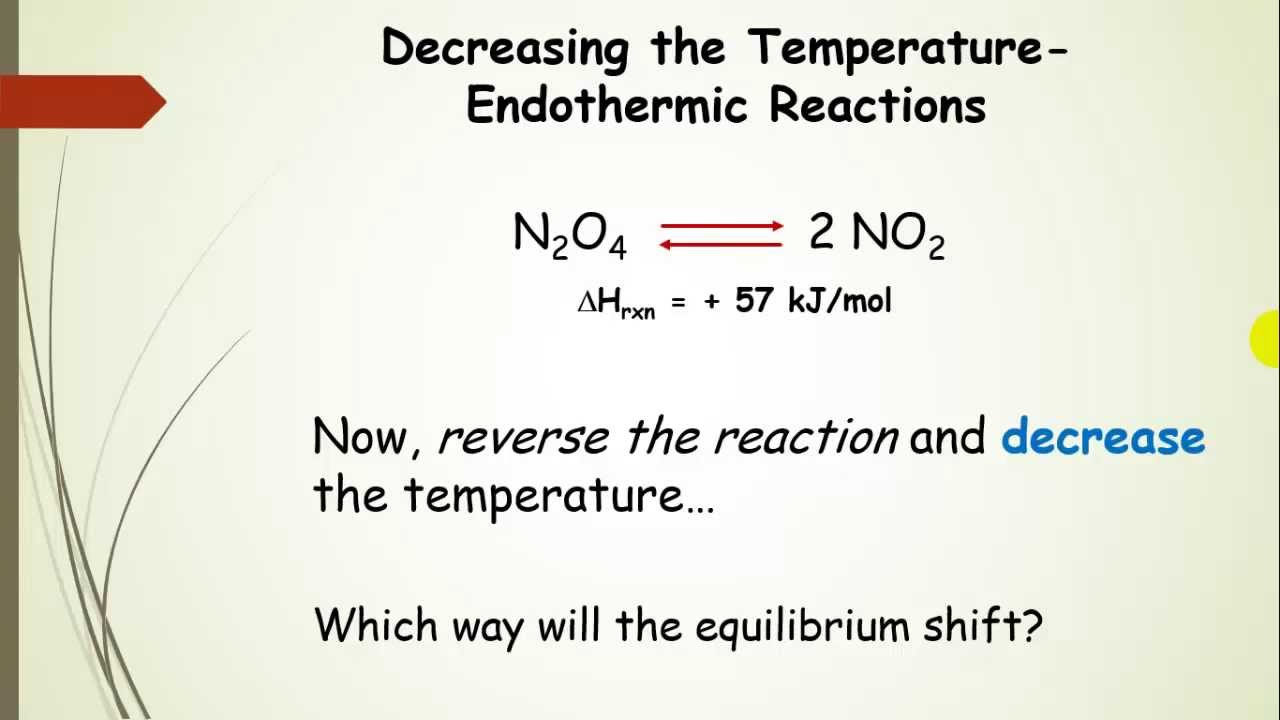
Endothermic Reaction Le Chatelier Principle . When a chemical system at equilibrium is disturbed, it returns to equilibrium by. le chatlier's principle says a system at equilibrium responds to changes in predictable ways. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and the other direction is an. le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature has on a system at. le chatelier's principle.
from www.youtube.com
le chatlier's principle says a system at equilibrium responds to changes in predictable ways. le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature has on a system at. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and the other direction is an. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. le chatelier's principle.
Le Chatelier's Principle and Temperature Changes (Pt. 10) YouTube
Endothermic Reaction Le Chatelier Principle the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and the other direction is an. le chatelier's principle. le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature has on a system at. le chatlier's principle says a system at equilibrium responds to changes in predictable ways.
From peakup.edu.vn
Le Chatelier’s Principle Peakup.edu.vn Endothermic Reaction Le Chatelier Principle le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature has on a system at. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. the back reaction (the conversion of c and d into a and b) would be. Endothermic Reaction Le Chatelier Principle.
From www.numerade.com
SOLVED Consider the following reaction at equilibrium 2 NH3 (g) = N2 Endothermic Reaction Le Chatelier Principle the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and the other direction is an. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. . Endothermic Reaction Le Chatelier Principle.
From www.numerade.com
SOLVED According to Le Chatelier s Principle; heat applied to an Endothermic Reaction Le Chatelier Principle le chatlier's principle says a system at equilibrium responds to changes in predictable ways. the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. le chatelier's principle. the back reaction (the conversion of. Endothermic Reaction Le Chatelier Principle.
From www.slideserve.com
PPT Section 8.4—Le Chatelier’s Principle PowerPoint Presentation ID Endothermic Reaction Le Chatelier Principle le chatlier's principle says a system at equilibrium responds to changes in predictable ways. le chatelier's principle. le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature has on a system at. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly. Endothermic Reaction Le Chatelier Principle.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID2170548 Endothermic Reaction Le Chatelier Principle in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and the other direction is an. le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature has on a system at. le chatlier's principle says a system at equilibrium responds to changes in predictable. Endothermic Reaction Le Chatelier Principle.
From www.numerade.com
SOLVEDUse Le Châtelier's principle and Kw in Table 61 to decide Endothermic Reaction Le Chatelier Principle the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. le chatlier's principle says a system at equilibrium responds to changes in predictable ways. in a reversible reaction, one direction is an exothermic reaction. Endothermic Reaction Le Chatelier Principle.
From www.youtube.com
Le Chatelier's Principle and Temperature Changes (Pt. 10) YouTube Endothermic Reaction Le Chatelier Principle le chatelier's principle. in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and the other direction is an. le chatlier's principle says a system at equilibrium responds to changes in predictable ways. le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature. Endothermic Reaction Le Chatelier Principle.
From slidetodoc.com
Le Chateliers Principle Main Concept Systems at equilibrium Endothermic Reaction Le Chatelier Principle the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. le chatelier's principle. le chatlier's principle says a system at equilibrium responds to changes in predictable ways. the back reaction (the conversion. Endothermic Reaction Le Chatelier Principle.
From schoolbag.info
FIGURE 15.13 Temperature and Le Châtelier's principle. Endothermic Reaction Le Chatelier Principle le chatelier's principle. the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and the other direction is an. le chatlier's principle says a system at equilibrium responds to. Endothermic Reaction Le Chatelier Principle.
From www.expii.com
Le Chatelier's Principle — Overview & Role in Equilibrium Expii Endothermic Reaction Le Chatelier Principle the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. le chatelier's principle. in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and the other. Endothermic Reaction Le Chatelier Principle.
From slideplayer.com
*Le Châtelier’s Principle and Equilibrium ppt download Endothermic Reaction Le Chatelier Principle the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature. Endothermic Reaction Le Chatelier Principle.
From www.slideserve.com
PPT Le Chatelier’s Principle PowerPoint Presentation, free download Endothermic Reaction Le Chatelier Principle in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and the other direction is an. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. le chatelier's principle. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly. Endothermic Reaction Le Chatelier Principle.
From demonstrations.wolfram.com
Le Chatelier's Principle in Chemical Equilibrium Wolfram Endothermic Reaction Le Chatelier Principle the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. le chatelier's principle. le chatlier's principle says a system at equilibrium responds to changes in. Endothermic Reaction Le Chatelier Principle.
From wisc.pb.unizin.org
Day 30 Le Châtelier’s Principle Chemistry 109 Endothermic Reaction Le Chatelier Principle the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. le chatelier's principle. le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature has on a system at. in a reversible reaction, one direction is an exothermic reaction (evolves heat. Endothermic Reaction Le Chatelier Principle.
From slideplayer.com
Chapter 6 Lecture Outline ppt download Endothermic Reaction Le Chatelier Principle the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. le chatelier's principle. le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature has on a system at. the back reaction (the conversion of c and d into a. Endothermic Reaction Le Chatelier Principle.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Endothermic Reaction Le Chatelier Principle le chatlier's principle says a system at equilibrium responds to changes in predictable ways. le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature has on a system at. le chatelier's principle. in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and. Endothermic Reaction Le Chatelier Principle.
From www.slideserve.com
PPT Le Chatelier’s Principle PowerPoint Presentation, free download Endothermic Reaction Le Chatelier Principle in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and the other direction is an. le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature has on a system at. le chatlier's principle says a system at equilibrium responds to changes in predictable. Endothermic Reaction Le Chatelier Principle.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Endothermic Reaction Le Chatelier Principle the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. le chatelier's principle. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. le chȃtelier’s principle can be used to predict the effect that a. Endothermic Reaction Le Chatelier Principle.
From slideplayer.com
The Position of Equilibrium ppt download Endothermic Reaction Le Chatelier Principle le chatlier's principle says a system at equilibrium responds to changes in predictable ways. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. in a reversible reaction, one direction is an exothermic. Endothermic Reaction Le Chatelier Principle.
From www.slideserve.com
PPT Collision Theory And Le Chatelier’s principle PowerPoint Endothermic Reaction Le Chatelier Principle le chatelier's principle. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. le chȃtelier’s principle can be used to predict the effect that a. Endothermic Reaction Le Chatelier Principle.
From www.wizeprep.com
Le Chatelier's Principle [more detail] Wize University Chemistry Endothermic Reaction Le Chatelier Principle in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and the other direction is an. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. le chatelier's principle. the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the. Endothermic Reaction Le Chatelier Principle.
From www.slideserve.com
PPT Le Chatelier’s Principle PowerPoint Presentation, free download Endothermic Reaction Le Chatelier Principle le chatelier's principle. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and the other. Endothermic Reaction Le Chatelier Principle.
From www.youtube.com
Everything you need to know about Le Chateliers Principle (Exothermic Endothermic Reaction Le Chatelier Principle le chatlier's principle says a system at equilibrium responds to changes in predictable ways. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. the back reaction (the conversion of c and d. Endothermic Reaction Le Chatelier Principle.
From slideplayer.com
Le Chatelier’s Principle ppt download Endothermic Reaction Le Chatelier Principle le chatlier's principle says a system at equilibrium responds to changes in predictable ways. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. le chȃtelier’s principle can be used to predict the. Endothermic Reaction Le Chatelier Principle.
From www.numerade.com
SOLVED Text Chapter 7 Chemical Equilibrium The Le Chatelier's Endothermic Reaction Le Chatelier Principle When a chemical system at equilibrium is disturbed, it returns to equilibrium by. the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. in a reversible. Endothermic Reaction Le Chatelier Principle.
From www.slideserve.com
PPT Reaction Rates and Le Chatelier’s Principle PowerPoint Endothermic Reaction Le Chatelier Principle the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature has on a system at. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. le chatelier's principle.. Endothermic Reaction Le Chatelier Principle.
From www.slideserve.com
PPT Le Chatelier’s Principle PowerPoint Presentation, free download Endothermic Reaction Le Chatelier Principle the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. le chȃtelier’s principle. Endothermic Reaction Le Chatelier Principle.
From slideplayer.com
Reaction Rates and Le Chatelier’s Principle ppt download Endothermic Reaction Le Chatelier Principle When a chemical system at equilibrium is disturbed, it returns to equilibrium by. le chatelier's principle. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature has on a system. Endothermic Reaction Le Chatelier Principle.
From www.numerade.com
SOLVED The formation of CoCL? from Co and Cl is endothermic Explain Endothermic Reaction Le Chatelier Principle the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature has on a system at. in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a. Endothermic Reaction Le Chatelier Principle.
From slideplayer.com
Le Chatelier’s Principle ppt download Endothermic Reaction Le Chatelier Principle in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and the other direction is an. the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. the back reaction (the conversion of c and d into a and b) would. Endothermic Reaction Le Chatelier Principle.
From inertgaszaimono.blogspot.com
Inert Gas Le Chatelier''s Principle Inert Gas Endothermic Reaction Le Chatelier Principle le chatelier's principle. le chatlier's principle says a system at equilibrium responds to changes in predictable ways. the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. in a reversible reaction, one direction. Endothermic Reaction Le Chatelier Principle.
From www.vedantu.com
Le Chatelier’s Principle Laws, Principle Example and FAQs for JEE Endothermic Reaction Le Chatelier Principle le chȃtelier’s principle can be used to predict the effect that a stress like changing temperature has on a system at. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. le chatlier's principle says a system at equilibrium responds to changes in predictable ways. le chatelier's principle. the back reaction (the conversion. Endothermic Reaction Le Chatelier Principle.
From www.slideserve.com
PPT Le Châtelier’s Principle PowerPoint Presentation, free download Endothermic Reaction Le Chatelier Principle in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and the other direction is an. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. . Endothermic Reaction Le Chatelier Principle.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Endothermic Reaction Le Chatelier Principle the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. the back reaction (the conversion of c and d into a and b) would be endothermic by exactly the same amount. When a chemical system at equilibrium is disturbed, it returns to equilibrium by. le chatelier's principle.. Endothermic Reaction Le Chatelier Principle.
From slidetodoc.com
Acids and Bases Todays topic Le Chateliers Principle Endothermic Reaction Le Chatelier Principle in a reversible reaction, one direction is an exothermic reaction (evolves heat and has a negative δh) and the other direction is an. the back reaction (the conversion of c and d into a and b) would be endothermic, absorbing the same amount. le chatlier's principle says a system at equilibrium responds to changes in predictable ways.. Endothermic Reaction Le Chatelier Principle.