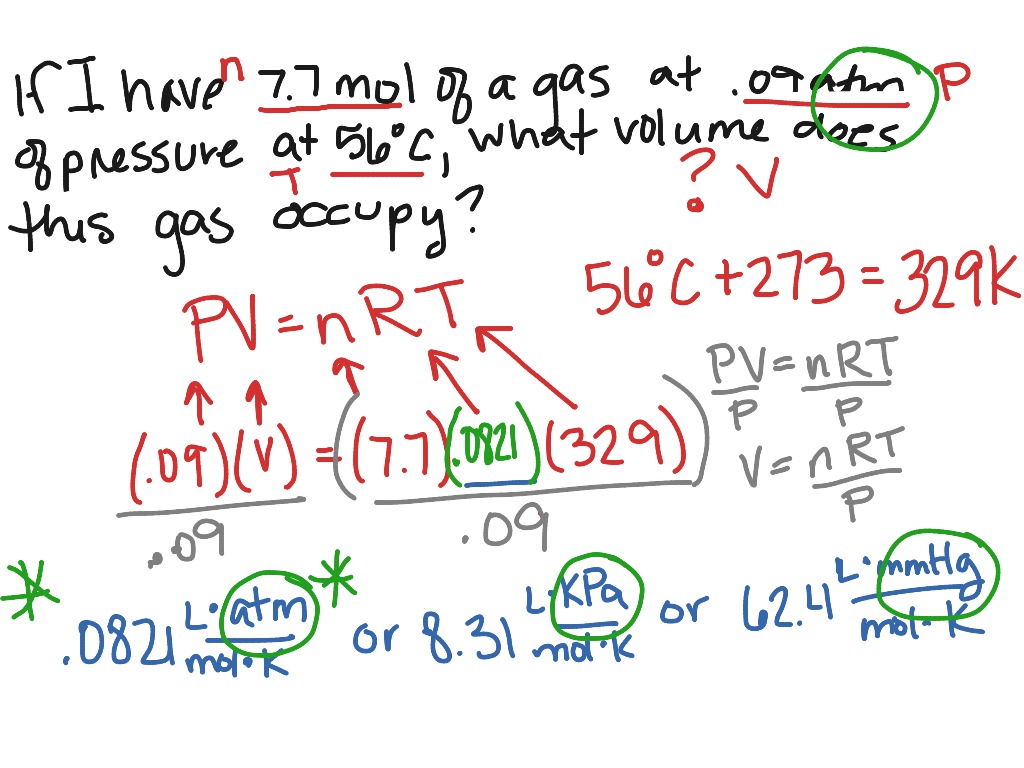
What Does R Mean In The Ideal Gas Law . Discover the definition and value of the gas constant. the ideal gas law. the gas constant or r is an essential constant in the ideal gas law. the four gas variables are: The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. Pv = nrt, where n is the number of moles of the gas and r is. in such a case, all gases obey an equation of state known as the ideal gas law:
from www.showme.com
Discover the definition and value of the gas constant. the ideal gas law. in such a case, all gases obey an equation of state known as the ideal gas law: the four gas variables are: Pressure (p), volume (v), number of mole of gas (n), and temperature (t). Pv = nrt, where n is the number of moles of the gas and r is. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. the gas constant or r is an essential constant in the ideal gas law.
Ideal gas law example Science, Chemistry, Gases ShowMe
What Does R Mean In The Ideal Gas Law in such a case, all gases obey an equation of state known as the ideal gas law: the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. the ideal gas law. Discover the definition and value of the gas constant. Pv = nrt, where n is the number of moles of the gas and r is. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. the gas constant or r is an essential constant in the ideal gas law. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). in such a case, all gases obey an equation of state known as the ideal gas law: the four gas variables are:
From poornima-kulkarni.blogspot.com
Ideal Gas Law R Values Solved The Ideal Gas Law Is Given By Pv = RT What Does R Mean In The Ideal Gas Law the ideal gas law. Pv = nrt, where n is the number of moles of the gas and r is. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the gas constant or r is an essential constant in the ideal gas law. the four gas variables are: the ideal gas law,. What Does R Mean In The Ideal Gas Law.
From www.slideshare.net
Ideal gas law practice mccpot What Does R Mean In The Ideal Gas Law Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the ideal gas law. in such a case, all gases obey an equation of state known as the ideal gas law: Pv = nrt, where n is the number of moles of the gas and r is. the four gas variables are: The volume. What Does R Mean In The Ideal Gas Law.
From erebusryling.blogspot.com
Ideal Gas Law R Values PPT Gas Laws PowerPoint Presentation ID What Does R Mean In The Ideal Gas Law the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. the ideal gas law. Discover the definition and value of the gas constant. in such a case, all gases. What Does R Mean In The Ideal Gas Law.
From plantecuador.com
Ideal Gas Law — Overview & Calculations Expii, ideal gas What Does R Mean In The Ideal Gas Law The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. the gas constant or r is an essential constant in the ideal gas law. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. Pv = nrt, where n is. What Does R Mean In The Ideal Gas Law.
From www.showme.com
Ideal gas law example Science, Chemistry, Gases ShowMe What Does R Mean In The Ideal Gas Law the ideal gas law. Pv = nrt, where n is the number of moles of the gas and r is. the gas constant or r is an essential constant in the ideal gas law. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. the four. What Does R Mean In The Ideal Gas Law.
From mmerevise.co.uk
The Ideal Gas Equation MME What Does R Mean In The Ideal Gas Law Pv = nrt, where n is the number of moles of the gas and r is. the gas constant or r is an essential constant in the ideal gas law. Discover the definition and value of the gas constant. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal. What Does R Mean In The Ideal Gas Law.
From www.sliderbase.com
The Ideal Gas Law Presentation Chemistry What Does R Mean In The Ideal Gas Law the four gas variables are: Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the gas constant or r is an essential constant in the ideal gas law. in such a case, all gases obey an equation of state known as the ideal gas law: The volume (v) occupied by n moles of. What Does R Mean In The Ideal Gas Law.
From www.youtube.com
Gas density and PV=nRT, the ideal gas law YouTube What Does R Mean In The Ideal Gas Law the gas constant or r is an essential constant in the ideal gas law. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. Discover the definition and value of the gas constant. Pv = nrt, where n is the number of moles of the gas and r is. the. What Does R Mean In The Ideal Gas Law.
From collegebookwormdiaries.blogspot.com
Different R Values Ideal Gas Law Create A Function To Convert R What Does R Mean In The Ideal Gas Law in such a case, all gases obey an equation of state known as the ideal gas law: Pv = nrt, where n is the number of moles of the gas and r is. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. the ideal gas law, also called the. What Does R Mean In The Ideal Gas Law.
From www.youtube.com
Master the Ideal Gas Law in Chemistry A StepbyStep Guide [1510 What Does R Mean In The Ideal Gas Law the ideal gas law. the gas constant or r is an essential constant in the ideal gas law. Discover the definition and value of the gas constant. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. Pv = nrt, where n is the number of moles. What Does R Mean In The Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download What Does R Mean In The Ideal Gas Law the four gas variables are: Pv = nrt, where n is the number of moles of the gas and r is. the gas constant or r is an essential constant in the ideal gas law. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. The volume. What Does R Mean In The Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law 01 PowerPoint Presentation, free download ID What Does R Mean In The Ideal Gas Law in such a case, all gases obey an equation of state known as the ideal gas law: the four gas variables are: Pressure (p), volume (v), number of mole of gas (n), and temperature (t). Discover the definition and value of the gas constant. The volume (v) occupied by n moles of any gas has a pressure (p). What Does R Mean In The Ideal Gas Law.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4387987 What Does R Mean In The Ideal Gas Law the four gas variables are: Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. in. What Does R Mean In The Ideal Gas Law.
From studylib.net
Ideal Gas Law What Does R Mean In The Ideal Gas Law The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the ideal gas law. in such a case, all gases obey an equation of state known as the ideal gas law: the gas constant or r. What Does R Mean In The Ideal Gas Law.
From studylib.net
IdealGas Equation The constant of proportionality is R What Does R Mean In The Ideal Gas Law Discover the definition and value of the gas constant. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). in such a case, all gases obey an equation of state known as the ideal gas law: The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. the. What Does R Mean In The Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law 01 PowerPoint Presentation, free download ID What Does R Mean In The Ideal Gas Law Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the gas constant or r is an essential constant in the ideal gas law. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. the four gas variables are: Discover the definition and value. What Does R Mean In The Ideal Gas Law.
From www.youtube.com
Introduction to the Ideal Gas Law Formula YouTube What Does R Mean In The Ideal Gas Law the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. in such a case, all gases obey an equation of state known as the ideal gas law: the gas constant or r is an essential constant in the ideal gas law. the ideal gas law. The. What Does R Mean In The Ideal Gas Law.
From einmalalle.blogspot.com
Ideal Gas Law R Values / Why are Gas Laws Important in Chemistry What Does R Mean In The Ideal Gas Law the gas constant or r is an essential constant in the ideal gas law. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. in such a case, all gases obey an equation of state known as the ideal gas law: Discover the definition and value of the gas constant.. What Does R Mean In The Ideal Gas Law.
From www.alamy.com
Ideal gas law, illustration Stock Photo Alamy What Does R Mean In The Ideal Gas Law Pv = nrt, where n is the number of moles of the gas and r is. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. in such a case, all gases obey an equation of state known as the ideal gas law: the ideal gas law. the ideal. What Does R Mean In The Ideal Gas Law.
From mungfali.com
Ideal Gas Law Chart What Does R Mean In The Ideal Gas Law the four gas variables are: Pressure (p), volume (v), number of mole of gas (n), and temperature (t). Pv = nrt, where n is the number of moles of the gas and r is. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. the gas constant. What Does R Mean In The Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID5758540 What Does R Mean In The Ideal Gas Law the gas constant or r is an essential constant in the ideal gas law. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). Pv = nrt, where n is the number of moles of the gas and r is. the ideal gas law. the ideal gas law, also called the general gas equation,. What Does R Mean In The Ideal Gas Law.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net What Does R Mean In The Ideal Gas Law The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. the gas constant or r is an essential constant in the ideal gas law. in such a case, all gases obey an equation of state known as the ideal gas law: the four gas variables are: the ideal. What Does R Mean In The Ideal Gas Law.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii What Does R Mean In The Ideal Gas Law in such a case, all gases obey an equation of state known as the ideal gas law: Discover the definition and value of the gas constant. Pv = nrt, where n is the number of moles of the gas and r is. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the four gas. What Does R Mean In The Ideal Gas Law.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID3344204 What Does R Mean In The Ideal Gas Law the ideal gas law. in such a case, all gases obey an equation of state known as the ideal gas law: Pv = nrt, where n is the number of moles of the gas and r is. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the gas constant or r is an. What Does R Mean In The Ideal Gas Law.
From byjus.com
Ideal Gas Law Equation Compressibility Of Natural Gas Chemistry What Does R Mean In The Ideal Gas Law Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the four gas variables are: Discover the definition and value of the gas constant. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. in such a case, all gases obey an equation of state known as. What Does R Mean In The Ideal Gas Law.
From www.britannica.com
Ideal gas law Definition, Formula, & Facts Britannica What Does R Mean In The Ideal Gas Law Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the four gas variables are: the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. the ideal gas law. the gas constant or r is an essential constant in the ideal gas law.. What Does R Mean In The Ideal Gas Law.
From sciencenotes.org
Ideal Gas Law Formula and Examples What Does R Mean In The Ideal Gas Law the ideal gas law. Pv = nrt, where n is the number of moles of the gas and r is. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the gas constant or r is an essential constant in the ideal gas law. The volume (v) occupied by n moles of any gas has. What Does R Mean In The Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 What Does R Mean In The Ideal Gas Law the gas constant or r is an essential constant in the ideal gas law. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. Discover the definition and value of the gas constant. the ideal gas law. The volume (v) occupied by n moles of any gas. What Does R Mean In The Ideal Gas Law.
From general.chemistrysteps.com
The Ideal Gas Law Chemistry Steps What Does R Mean In The Ideal Gas Law the ideal gas law. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. Discover the definition and value of the gas constant. the four gas variables are: in such a case, all gases obey an equation of state known as the ideal gas law: . What Does R Mean In The Ideal Gas Law.
From www.slideserve.com
PPT The ideal gas law PowerPoint Presentation, free download ID3608170 What Does R Mean In The Ideal Gas Law Discover the definition and value of the gas constant. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. the four gas variables are: the ideal gas law. the gas constant or r is an essential constant in the ideal gas law. in such a case, all gases. What Does R Mean In The Ideal Gas Law.
From www.slideserve.com
PPT Chapter 5 Gases PowerPoint Presentation, free download ID593425 What Does R Mean In The Ideal Gas Law the gas constant or r is an essential constant in the ideal gas law. in such a case, all gases obey an equation of state known as the ideal gas law: Pv = nrt, where n is the number of moles of the gas and r is. the ideal gas law. Pressure (p), volume (v), number of. What Does R Mean In The Ideal Gas Law.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4387987 What Does R Mean In The Ideal Gas Law the gas constant or r is an essential constant in the ideal gas law. Pv = nrt, where n is the number of moles of the gas and r is. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). in such a case, all gases obey an equation of state known as the ideal. What Does R Mean In The Ideal Gas Law.
From www.youtube.com
AP Chemistry Ideal Gas Law YouTube What Does R Mean In The Ideal Gas Law in such a case, all gases obey an equation of state known as the ideal gas law: Pv = nrt, where n is the number of moles of the gas and r is. the gas constant or r is an essential constant in the ideal gas law. The volume (v) occupied by n moles of any gas has. What Does R Mean In The Ideal Gas Law.
From www.youtube.com
Ideal Gas Equation How to Choose the Correct Gas Constant, R? With What Does R Mean In The Ideal Gas Law Pv = nrt, where n is the number of moles of the gas and r is. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. the gas constant or r is an essential constant in the ideal gas law. the ideal gas law. Pressure (p), volume. What Does R Mean In The Ideal Gas Law.
From www.slideserve.com
PPT C H A P T E R 14 The Ideal Gas Law and Theory PowerPoint What Does R Mean In The Ideal Gas Law the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. the ideal gas law. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. Pv = nrt,. What Does R Mean In The Ideal Gas Law.