Kmno4 Fe2+ Titration Equation . A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. determination of %fe in an ore.
from www.slideserve.com
A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. determination of %fe in an ore. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores.
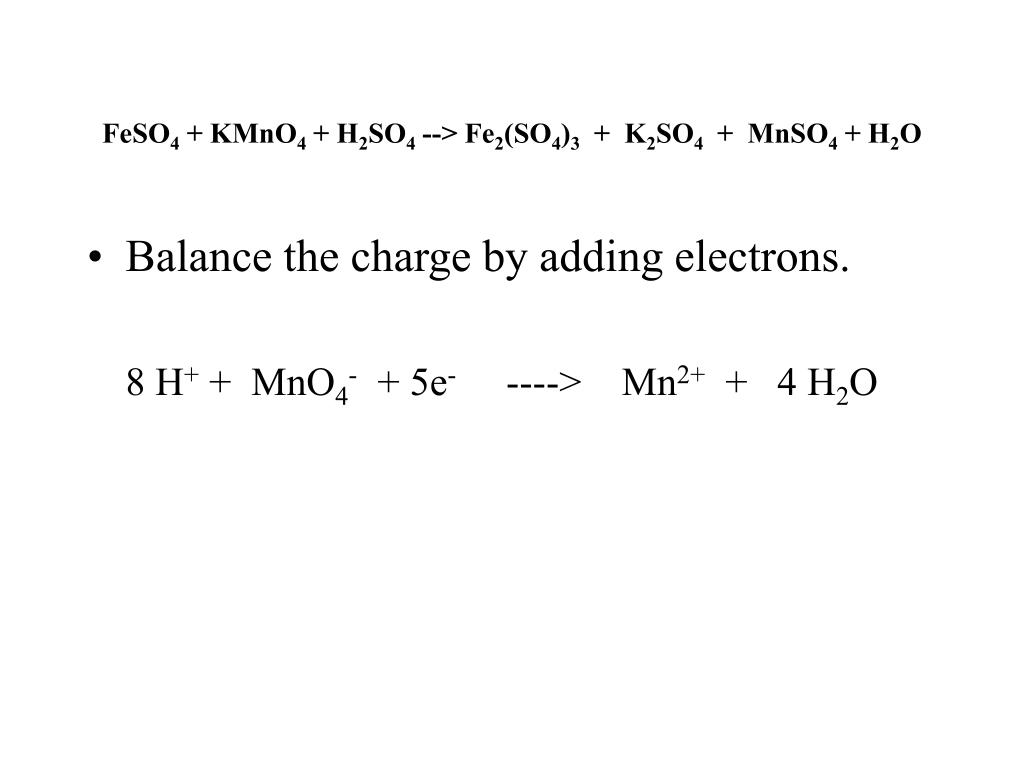
PPT Balancing Redox Reactions Carol Brown Saint Mary’s Hall PowerPoint Presentation ID4350533
Kmno4 Fe2+ Titration Equation determination of %fe in an ore. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. determination of %fe in an ore.
From www.youtube.com
Ammonium Iron (II) Sulphate vs KMnO4 Titration Calculations Example YouTube Kmno4 Fe2+ Titration Equation determination of %fe in an ore. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. the redox titration of permanganate. Kmno4 Fe2+ Titration Equation.
From www.youtube.com
titration of kmno4 with mohr's salt class 12 determine the concentration of KMnO4 using mohr Kmno4 Fe2+ Titration Equation the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. determination of %fe in an ore. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced. Kmno4 Fe2+ Titration Equation.
From www.numerade.com
SOLVED Using the halfredox method, what is the balanced redox equation of a. KMnO4 and Na2C2O4 Kmno4 Fe2+ Titration Equation the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. the redox titration of permanganate ions is commonly used to determine the. Kmno4 Fe2+ Titration Equation.
From www.congress-intercultural.eu
To Determine The Molarity Of KMnO4 Solution By Titrating It, 44 OFF Kmno4 Fe2+ Titration Equation determination of %fe in an ore. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. the redox titration of permanganate. Kmno4 Fe2+ Titration Equation.
From www.youtube.com
General Chemistry Standardization of KMnO4 By Redox Titration YouTube Kmno4 Fe2+ Titration Equation the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. determination of %fe in an ore. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and. Kmno4 Fe2+ Titration Equation.
From www.scribd.com
4 Estimation of Fe2 by KMnO4 PDF Titration Chemistry Kmno4 Fe2+ Titration Equation this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. the kmno4 solution (about 0.02m) is first standardized. Kmno4 Fe2+ Titration Equation.
From slideplayer.com
Balance the following MnO4 Mn2+ Fe2+ Fe ppt download Kmno4 Fe2+ Titration Equation the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. this video demonstrates the. Kmno4 Fe2+ Titration Equation.
From www.numerade.com
SOLVED Write the Redox titration report for determination of Fe2+ concentration by Potassium Kmno4 Fe2+ Titration Equation this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. determination of %fe in an ore. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. the redox titration of permanganate. Kmno4 Fe2+ Titration Equation.
From www.numerade.com
SOLVED The iron content of drinking water can be measured by titration with KMnO4. The reaction Kmno4 Fe2+ Titration Equation A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. determination of %fe in. Kmno4 Fe2+ Titration Equation.
From www.numerade.com
SOLVED Redox Titration Potassium permanganate, KMnO4, is a strong oxidizing agent. MnO4 is an Kmno4 Fe2+ Titration Equation A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. determination of %fe in an ore. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the. Kmno4 Fe2+ Titration Equation.
From www.numerade.com
SOLVED The KMnO4 solution is first standardized by titration using Mohr's salt; ferrous Kmno4 Fe2+ Titration Equation the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the kmno4 solution (about 0.02m) is first standardized. Kmno4 Fe2+ Titration Equation.
From hamdahalsten.blogspot.com
46+ calculate the mass percent of oxygen in kmno4 HamdaHalsten Kmno4 Fe2+ Titration Equation determination of %fe in an ore. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate. Kmno4 Fe2+ Titration Equation.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID2952040 Kmno4 Fe2+ Titration Equation the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. determination of %fe in. Kmno4 Fe2+ Titration Equation.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 Kmno4 Fe2+ Titration Equation the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. determination of %fe in an ore. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium. Kmno4 Fe2+ Titration Equation.
From slideplayer.com
Lesson 6 Redox Titrations ppt download Kmno4 Fe2+ Titration Equation determination of %fe in an ore. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. the redox titration of permanganate. Kmno4 Fe2+ Titration Equation.
From www.youtube.com
Standardization of KMnO4 Redox Titration KMnO4 Vs Fe2+ Mohrs salt titration YouTube Kmno4 Fe2+ Titration Equation A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. the redox titration of permanganate ions is commonly used to determine the. Kmno4 Fe2+ Titration Equation.
From www.youtube.com
FeC2O4+KMnO4+H2SO4 GIVES Fe2(SO4)3+MnSO4+ K2S04+H2O+CO2. Balancing Redox reaction YouTube Kmno4 Fe2+ Titration Equation A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. determination of %fe in. Kmno4 Fe2+ Titration Equation.
From exoyrpuml.blob.core.windows.net
Titration Of Kmno4 With Sodium Oxalate at Mike Stevens blog Kmno4 Fe2+ Titration Equation the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the redox titration of permanganate ions is commonly used to determine the. Kmno4 Fe2+ Titration Equation.
From androidtraining.metmans.edu.eg
FeSO4 KMnO4 H2SO4 Fe2(SO4)3 MnSO4 H2O K2SO4 Tuition Tube, 42 OFF Kmno4 Fe2+ Titration Equation this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. determination of %fe in an ore. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate. Kmno4 Fe2+ Titration Equation.
From www.numerade.com
SOLVED The Fe2+ content of iron tablets was determined by titration with a freshly standardized Kmno4 Fe2+ Titration Equation the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the kmno4 solution (about 0.02m) is first standardized. Kmno4 Fe2+ Titration Equation.
From www.numerade.com
SOLVED Repeat the titration at least two additional times, recording your data in the data Kmno4 Fe2+ Titration Equation determination of %fe in an ore. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and. Kmno4 Fe2+ Titration Equation.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID4545121 Kmno4 Fe2+ Titration Equation this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. determination of %fe in an ore. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron. Kmno4 Fe2+ Titration Equation.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Kmno4 Fe2+ Titration Equation this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. determination of %fe in an ore. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron. Kmno4 Fe2+ Titration Equation.
From www.numerade.com
SOLVED86. So.00mL sample of solution containing Fe2t ions is titrated with 0.0216 M KMnO4 Kmno4 Fe2+ Titration Equation A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. determination of %fe in an ore. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the. Kmno4 Fe2+ Titration Equation.
From www.slideserve.com
PPT The Breathalyzer PowerPoint Presentation, free download ID5480133 Kmno4 Fe2+ Titration Equation the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. determination of %fe in an ore. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. the redox titration of permanganate. Kmno4 Fe2+ Titration Equation.
From www.numerade.com
SOLVED In the titration of ammonium iron (II) sulfate hexahydrate with a standard solution of Kmno4 Fe2+ Titration Equation the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. this video demonstrates the. Kmno4 Fe2+ Titration Equation.
From www.numerade.com
SOLVED A standardized 0.153 mol/L solution of KMnO4(aq) was used to titrate 10.00 mL samples of Kmno4 Fe2+ Titration Equation the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. determination of %fe in an ore. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium. Kmno4 Fe2+ Titration Equation.
From www.youtube.com
Titration KMnO4 Vs Mohr Salt in Hindi Full Experiment + Calculations Chemistry Practical Kmno4 Fe2+ Titration Equation the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. determination of %fe in an ore. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the. Kmno4 Fe2+ Titration Equation.
From www.youtube.com
How to Balance KMnO4 + Fe = FeO + K2O + MnO2 YouTube Kmno4 Fe2+ Titration Equation this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. determination of %fe in an. Kmno4 Fe2+ Titration Equation.
From www.numerade.com
SOLVEDThe percent iron in iron ore can be determined by dissolving the ore in acid, then Kmno4 Fe2+ Titration Equation A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. determination of %fe in an ore. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the. Kmno4 Fe2+ Titration Equation.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Kmno4 Fe2+ Titration Equation the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. A 0.4857 g iron ore sample. Kmno4 Fe2+ Titration Equation.
From www.youtube.com
Titration Of Kmno4 Vs Oxalic Acid The Ultimate Guide Titration KMnO4 vs oxalic acid Kmno4 Fe2+ Titration Equation this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. the redox titration of permanganate ions is commonly used to determine the. Kmno4 Fe2+ Titration Equation.
From www.numerade.com
SOLVEDRedox Titrationi Acidic Medium Puch Slider Upto Add Volume of KMnO Tetal Volume of KMno4 Kmno4 Fe2+ Titration Equation the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. A 0.4857 g iron ore sample. Kmno4 Fe2+ Titration Equation.
From www.slideserve.com
PPT Balancing Redox Reactions Carol Brown Saint Mary’s Hall PowerPoint Presentation ID4350533 Kmno4 Fe2+ Titration Equation determination of %fe in an ore. the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. this video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. the redox titration of permanganate. Kmno4 Fe2+ Titration Equation.
From www.chegg.com
Solved Standardization of KMnO4 We permormed a titration Kmno4 Fe2+ Titration Equation the kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. A 0.4857 g iron ore sample was dissolved in concentrated acid and reduced to fe2+. determination of %fe in. Kmno4 Fe2+ Titration Equation.