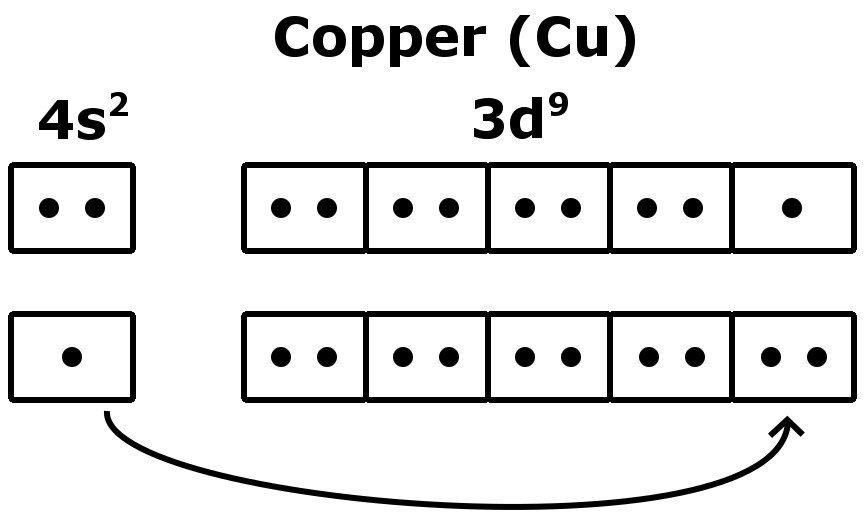
Copper Atom Unpaired Electrons . one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron configuration. these elements have still got an unpaired electron. Because all the 2 p orbitals are degenerate, it doesn’t matter which one has the pair of electrons. an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired electrons means all two. in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. One can write an electronic configuration that. the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2 3p6 3d10 4s1) an unpaired electron is an electron that occupies. when assigning electrons to orbitals, an electron first seeks to fill all the orbitals with similar energy (also referred to as. But it's so negligible that. Copper is an element that has an atomic number $ {\text {29}}$. the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the.
from periodictable.me
But it's so negligible that. the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the. an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired electrons means all two. Because all the 2 p orbitals are degenerate, it doesn’t matter which one has the pair of electrons. one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron configuration. Copper is an element that has an atomic number $ {\text {29}}$. the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2 3p6 3d10 4s1) an unpaired electron is an electron that occupies. in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. One can write an electronic configuration that. when assigning electrons to orbitals, an electron first seeks to fill all the orbitals with similar energy (also referred to as.
How To Find A Electron Configuration For Copper Dynamic Periodic
Copper Atom Unpaired Electrons One can write an electronic configuration that. an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired electrons means all two. the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2 3p6 3d10 4s1) an unpaired electron is an electron that occupies. these elements have still got an unpaired electron. Copper is an element that has an atomic number $ {\text {29}}$. when assigning electrons to orbitals, an electron first seeks to fill all the orbitals with similar energy (also referred to as. Because all the 2 p orbitals are degenerate, it doesn’t matter which one has the pair of electrons. But it's so negligible that. the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the. one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron configuration. One can write an electronic configuration that. in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Atom Unpaired Electrons Copper is an element that has an atomic number $ {\text {29}}$. an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired electrons means all two. One can write an electronic configuration that. in chemistry, an unpaired electron is an electron that occupies an orbital. Copper Atom Unpaired Electrons.
From www.alamy.com
Symbol and electron diagram for Copper Stock Vector Image & Art Alamy Copper Atom Unpaired Electrons when assigning electrons to orbitals, an electron first seeks to fill all the orbitals with similar energy (also referred to as. one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron configuration. Copper is an element that has an. Copper Atom Unpaired Electrons.
From www.dreamstime.com
Atom of Copper with Detailed Core and Its 29 Electrons with Atoms Stock Copper Atom Unpaired Electrons Copper is an element that has an atomic number $ {\text {29}}$. in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. But it's so negligible that. when assigning electrons to orbitals, an electron first seeks to fill all the orbitals with similar energy (also. Copper Atom Unpaired Electrons.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration Copper Atom Unpaired Electrons in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. these elements have still got an unpaired electron. Because all the 2 p orbitals are degenerate, it doesn’t matter which one has the pair of electrons. Copper is an element that has an atomic number. Copper Atom Unpaired Electrons.
From www.alamy.com
Symbol and electron diagram for Copper illustration Stock Vector Image Copper Atom Unpaired Electrons Copper is an element that has an atomic number $ {\text {29}}$. But it's so negligible that. Because all the 2 p orbitals are degenerate, it doesn’t matter which one has the pair of electrons. One can write an electronic configuration that. the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2 3p6 3d10 4s1) an. Copper Atom Unpaired Electrons.
From elchoroukhost.net
Copper Periodic Table Valence Electrons Elcho Table Copper Atom Unpaired Electrons One can write an electronic configuration that. when assigning electrons to orbitals, an electron first seeks to fill all the orbitals with similar energy (also referred to as. the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the. these elements have still got an unpaired electron. Copper is. Copper Atom Unpaired Electrons.
From mungfali.com
Orbital Diagram Of Copper Copper Atom Unpaired Electrons the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2 3p6 3d10 4s1) an unpaired electron is an electron that occupies. One can write an electronic configuration that. an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired electrons means all two.. Copper Atom Unpaired Electrons.
From www.shutterstock.com
Bohr Model Copper Atom Electron Structure Stock Vector (Royalty Free Copper Atom Unpaired Electrons One can write an electronic configuration that. the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2 3p6 3d10 4s1) an unpaired electron is an electron that occupies. in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. an unpaired electron. Copper Atom Unpaired Electrons.
From www.earthdate.org
Copper’s Superpower EarthDate Copper Atom Unpaired Electrons when assigning electrons to orbitals, an electron first seeks to fill all the orbitals with similar energy (also referred to as. one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron configuration. Because all the 2 p orbitals are. Copper Atom Unpaired Electrons.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Copper Atom Unpaired Electrons one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron configuration. Copper is an element that has an atomic number $ {\text {29}}$. Because all the 2 p orbitals are degenerate, it doesn’t matter which one has the pair of. Copper Atom Unpaired Electrons.
From www.dreamstime.com
Electron of the Element Copper Stock Vector Illustration of Copper Atom Unpaired Electrons in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. One can write an electronic configuration that. But it's so negligible that. an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired. Copper Atom Unpaired Electrons.
From ar.inspiredpencil.com
Orbital Diagram For Copper Copper Atom Unpaired Electrons Copper is an element that has an atomic number $ {\text {29}}$. an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired electrons means all two. One can write an electronic configuration that. the electron configuration of copper is color (blue) ( [1s2 2s2 2p6. Copper Atom Unpaired Electrons.
From www.alamy.com
Symbol and electron diagram of Copper illustration Stock Vector Image Copper Atom Unpaired Electrons an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired electrons means all two. But it's so negligible that. when assigning electrons to orbitals, an electron first seeks to fill all the orbitals with similar energy (also referred to as. Copper is an element that. Copper Atom Unpaired Electrons.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Atom Unpaired Electrons in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. Copper is an element that has an atomic number $ {\text {29}}$. the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the. One can write an. Copper Atom Unpaired Electrons.
From www.dreamstime.com
Atom of Copper with Detailed Core and Its 29 Electrons on Black Stock Copper Atom Unpaired Electrons the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the. in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. when assigning electrons to orbitals, an electron first seeks to fill all the orbitals with. Copper Atom Unpaired Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Copper Atom Unpaired Electrons an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired electrons means all two. the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2 3p6 3d10 4s1) an unpaired electron is an electron that occupies. these elements have still got an. Copper Atom Unpaired Electrons.
From childhealthpolicy.vumc.org
💐 Copper atom model. Copper Particles. 20221017 Copper Atom Unpaired Electrons the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2 3p6 3d10 4s1) an unpaired electron is an electron that occupies. an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired electrons means all two. one electron must be paired with. Copper Atom Unpaired Electrons.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition and electron Copper Atom Unpaired Electrons One can write an electronic configuration that. an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired electrons means all two. Copper is an element that has an atomic number $ {\text {29}}$. in chemistry, an unpaired electron is an electron that occupies an orbital. Copper Atom Unpaired Electrons.
From elchoroukhost.net
Copper Periodic Table Protons Neutrons And Electrons Elcho Table Copper Atom Unpaired Electrons one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron configuration. But it's so negligible that. these elements have still got an unpaired electron. Because all the 2 p orbitals are degenerate, it doesn’t matter which one has the. Copper Atom Unpaired Electrons.
From www.sciencephoto.com
Copper, atomic structure Stock Image C013/1552 Science Photo Library Copper Atom Unpaired Electrons the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2 3p6 3d10 4s1) an unpaired electron is an electron that occupies. the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the. Copper is an element that has an atomic number $ {\text {29}}$. But it's so. Copper Atom Unpaired Electrons.
From ar.inspiredpencil.com
Copper Atom Model Copper Atom Unpaired Electrons in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. One can write an electronic configuration that. But it's so negligible that. these elements have still got an unpaired electron. the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2 3p6. Copper Atom Unpaired Electrons.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Copper Atom Unpaired Electrons the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2 3p6 3d10 4s1) an unpaired electron is an electron that occupies. Copper is an element that has an atomic number $ {\text {29}}$. the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the. Because all the. Copper Atom Unpaired Electrons.
From techiescientist.com
Is Copper an Element? Techiescientist Copper Atom Unpaired Electrons these elements have still got an unpaired electron. in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. Because all the 2 p orbitals are degenerate, it doesn’t matter which one has the pair of electrons. when assigning electrons to orbitals, an electron first. Copper Atom Unpaired Electrons.
From www.youtube.com
ELECTRONIC CONFIGURATION OF COPPER ATOM and STABILITY YouTube Copper Atom Unpaired Electrons But it's so negligible that. one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron configuration. the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2 3p6 3d10 4s1) an unpaired electron is an electron that. Copper Atom Unpaired Electrons.
From ar.inspiredpencil.com
Copper Orbital Diagram Copper Atom Unpaired Electrons But it's so negligible that. one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron configuration. when assigning electrons to orbitals, an electron first seeks to fill all the orbitals with similar energy (also referred to as. in. Copper Atom Unpaired Electrons.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Copper Atom Unpaired Electrons an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired electrons means all two. Because all the 2 p orbitals are degenerate, it doesn’t matter which one has the pair of electrons. the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2. Copper Atom Unpaired Electrons.
From valenceelectrons.com
Copper(Cu) electron configuration and orbital diagram Copper Atom Unpaired Electrons One can write an electronic configuration that. these elements have still got an unpaired electron. the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2 3p6 3d10 4s1) an unpaired electron is an electron that occupies. one electron must be paired with another in one of the 2 p orbitals, which gives us two. Copper Atom Unpaired Electrons.
From www.nuclear-power.com
Copper Electron Affinity Electronegativity Ionization Energy of Copper Atom Unpaired Electrons an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired electrons means all two. the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the. the electron configuration of copper is color (blue) ( [1s2 2s2. Copper Atom Unpaired Electrons.
From www.youtube.com
How To Determine The Number of Paired and Unpaired Electrons YouTube Copper Atom Unpaired Electrons an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired electrons means all two. Copper is an element that has an atomic number $ {\text {29}}$. these elements have still got an unpaired electron. one electron must be paired with another in one of. Copper Atom Unpaired Electrons.
From www.slideshare.net
Chapter 6 Lecture Electrons in Atoms Copper Atom Unpaired Electrons One can write an electronic configuration that. when assigning electrons to orbitals, an electron first seeks to fill all the orbitals with similar energy (also referred to as. one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron configuration.. Copper Atom Unpaired Electrons.
From www.slideserve.com
PPT Chapter 10 Basic Concepts of Chemical Bonding PowerPoint Copper Atom Unpaired Electrons these elements have still got an unpaired electron. Because all the 2 p orbitals are degenerate, it doesn’t matter which one has the pair of electrons. One can write an electronic configuration that. when assigning electrons to orbitals, an electron first seeks to fill all the orbitals with similar energy (also referred to as. an unpaired electron. Copper Atom Unpaired Electrons.
From www.mooramo.com
Ions of Transition Elements Mooramo Copper Atom Unpaired Electrons in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. these elements have still got an unpaired electron. an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair (paired electrons means all. Copper Atom Unpaired Electrons.
From material-properties.org
Copper Periodic Table and Atomic Properties Copper Atom Unpaired Electrons Copper is an element that has an atomic number $ {\text {29}}$. the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2 3p6 3d10 4s1) an unpaired electron is an electron that occupies. in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an. Copper Atom Unpaired Electrons.
From sites.google.com
Unit 4 Lewis Dot Structures & Bonding Mrosla Science Copper Atom Unpaired Electrons Because all the 2 p orbitals are degenerate, it doesn’t matter which one has the pair of electrons. the electron configuration of copper is color (blue) ( [1s2 2s2 2p6 3s2 3p6 3d10 4s1) an unpaired electron is an electron that occupies. one electron must be paired with another in one of the 2 p orbitals, which gives. Copper Atom Unpaired Electrons.
From www.alamy.com
3d render of atom structure of copper isolated over white background Copper Atom Unpaired Electrons one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron configuration. Because all the 2 p orbitals are degenerate, it doesn’t matter which one has the pair of electrons. One can write an electronic configuration that. these elements have. Copper Atom Unpaired Electrons.