Titration Of Hcl With Naoh Lab Report . preparation of a standard sodium hydroxide solution and titration of hydrochloric acid. there are two primary factors that determine the extent of injury due to exposure to corrosive chemicals like naoh, the concentration of the. Since 1 mole of naoh. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In this experiment, we prepare solutions of. the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with. To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m. the volume of naoh solution required to react with a known weight of khp is determined by titration.
from mungfali.com
the volume of naoh solution required to react with a known weight of khp is determined by titration. To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m. the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with. there are two primary factors that determine the extent of injury due to exposure to corrosive chemicals like naoh, the concentration of the. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In this experiment, we prepare solutions of. preparation of a standard sodium hydroxide solution and titration of hydrochloric acid. Since 1 mole of naoh.
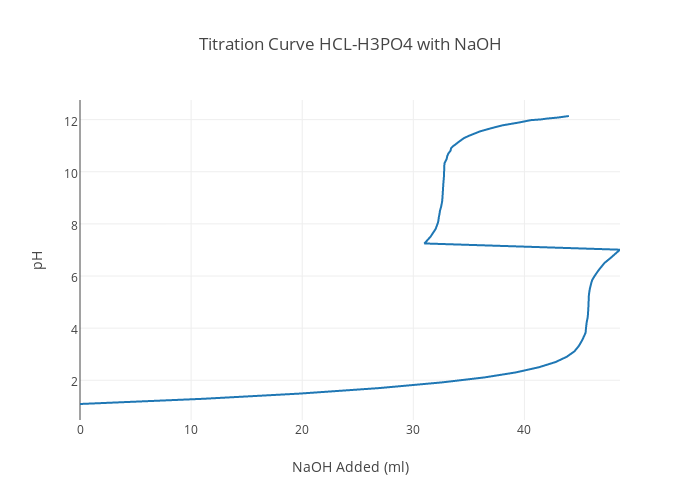
NaOH HCl Titration Curve
Titration Of Hcl With Naoh Lab Report the volume of naoh solution required to react with a known weight of khp is determined by titration. preparation of a standard sodium hydroxide solution and titration of hydrochloric acid. Since 1 mole of naoh. the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with. the volume of naoh solution required to react with a known weight of khp is determined by titration. In this experiment, we prepare solutions of. there are two primary factors that determine the extent of injury due to exposure to corrosive chemicals like naoh, the concentration of the. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m.
From www.chegg.com
Solved Titration of HCl with standardized NaOH Titration Of Hcl With Naoh Lab Report To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m. Since 1 mole of naoh. the volume of naoh solution required to react with a known weight of khp is determined by titration. there are two primary factors that determine the. Titration Of Hcl With Naoh Lab Report.
From www.youtube.com
Classic Titration of HCl with NaOH YouTube Titration Of Hcl With Naoh Lab Report In this experiment, we prepare solutions of. there are two primary factors that determine the extent of injury due to exposure to corrosive chemicals like naoh, the concentration of the. preparation of a standard sodium hydroxide solution and titration of hydrochloric acid. one type of titration uses a neutralization reaction, in which an acid and a base. Titration Of Hcl With Naoh Lab Report.
From studylib.net
Acid Base Titration Lab Titration Of Hcl With Naoh Lab Report one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with. preparation of a standard sodium hydroxide solution and titration of hydrochloric acid.. Titration Of Hcl With Naoh Lab Report.
From www.chegg.com
Solved Experiment Estimation of sodiumhydroxide using Titration Of Hcl With Naoh Lab Report Since 1 mole of naoh. the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with. the volume of naoh solution required to react with a known weight of khp is determined by titration. preparation of a standard sodium hydroxide solution and titration of hydrochloric. Titration Of Hcl With Naoh Lab Report.
From www.studocu.com
Hcl naoh lab lab report Chem 1252, General Chemistry I Lab Johnson Titration Of Hcl With Naoh Lab Report To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m. Since 1 mole of naoh. preparation of a standard sodium hydroxide solution and titration of hydrochloric acid. there are two primary factors that determine the extent of injury due to exposure. Titration Of Hcl With Naoh Lab Report.
From www.slideshare.net
Titration experiment report Titration Of Hcl With Naoh Lab Report preparation of a standard sodium hydroxide solution and titration of hydrochloric acid. the volume of naoh solution required to react with a known weight of khp is determined by titration. Since 1 mole of naoh. In this experiment, we prepare solutions of. the purpose of the titration is to find the concentration of the hydrochloric acid with. Titration Of Hcl With Naoh Lab Report.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Titration Of Hcl With Naoh Lab Report In this experiment, we prepare solutions of. the volume of naoh solution required to react with a known weight of khp is determined by titration. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: preparation of a standard sodium hydroxide solution and titration of. Titration Of Hcl With Naoh Lab Report.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Titration Of Hcl With Naoh Lab Report To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m. the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with. preparation of a standard sodium hydroxide solution and. Titration Of Hcl With Naoh Lab Report.
From mungfali.com
NaOH HCl Titration Curve Titration Of Hcl With Naoh Lab Report To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m. the volume of naoh solution required to react with a known weight of khp is determined by titration. In this experiment, we prepare solutions of. there are two primary factors that. Titration Of Hcl With Naoh Lab Report.
From mungfali.com
HCl NaOH Titration Titration Of Hcl With Naoh Lab Report In this experiment, we prepare solutions of. preparation of a standard sodium hydroxide solution and titration of hydrochloric acid. Since 1 mole of naoh. there are two primary factors that determine the extent of injury due to exposure to corrosive chemicals like naoh, the concentration of the. the volume of naoh solution required to react with a. Titration Of Hcl With Naoh Lab Report.
From www.numerade.com
SOLVED Write the laboratory report for determination of NaOH Titration Of Hcl With Naoh Lab Report preparation of a standard sodium hydroxide solution and titration of hydrochloric acid. To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a. Titration Of Hcl With Naoh Lab Report.
From studylib.net
Titration of HCl with NaOH Titration Of Hcl With Naoh Lab Report the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with. the volume of naoh solution required to react with a known weight of khp is determined by titration. In this experiment, we prepare solutions of. preparation of a standard sodium hydroxide solution and titration. Titration Of Hcl With Naoh Lab Report.
From snipe.fm
️ Standardization of hcl and naoh. Titrating sodium hydroxide with Titration Of Hcl With Naoh Lab Report the volume of naoh solution required to react with a known weight of khp is determined by titration. there are two primary factors that determine the extent of injury due to exposure to corrosive chemicals like naoh, the concentration of the. Since 1 mole of naoh. one type of titration uses a neutralization reaction, in which an. Titration Of Hcl With Naoh Lab Report.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Titration Of Hcl With Naoh Lab Report the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with. there are two primary factors that determine the extent of injury due to exposure to corrosive chemicals like naoh, the concentration of the. one type of titration uses a neutralization reaction, in which an. Titration Of Hcl With Naoh Lab Report.
From pubs.sciepub.com
Figure 5B. Plot of the titration of strong acid (HCl= 0.1M) with strong Titration Of Hcl With Naoh Lab Report there are two primary factors that determine the extent of injury due to exposure to corrosive chemicals like naoh, the concentration of the. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: the volume of naoh solution required to react with a known weight. Titration Of Hcl With Naoh Lab Report.
From www.studocu.com
Lab report Titration of HCl with NaOH Prelab Title Titration of Titration Of Hcl With Naoh Lab Report Since 1 mole of naoh. To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m. the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with. In this experiment, we. Titration Of Hcl With Naoh Lab Report.
From kyrakruwesparza.blogspot.com
Acidbase Titration Lab Report Answers Hcl and Naoh KyrakruwEsparza Titration Of Hcl With Naoh Lab Report Since 1 mole of naoh. the volume of naoh solution required to react with a known weight of khp is determined by titration. the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with. To determine the precision of the titration of hcl with naoh by. Titration Of Hcl With Naoh Lab Report.
From www.studypool.com
SOLUTION Laboratory Report Standardization of NaOH with KHP and Titration Of Hcl With Naoh Lab Report Since 1 mole of naoh. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: preparation of a standard sodium hydroxide solution and titration of hydrochloric acid. the purpose of the titration is to find the concentration of the hydrochloric acid with the help of. Titration Of Hcl With Naoh Lab Report.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Titration Of Hcl With Naoh Lab Report In this experiment, we prepare solutions of. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Since 1 mole of naoh. To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a. Titration Of Hcl With Naoh Lab Report.
From byjus.com
The graph of pH during the titration of NaOH and HCl Titration Of Hcl With Naoh Lab Report Since 1 mole of naoh. the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with. the volume of naoh solution required to react with a known weight of khp is determined by titration. preparation of a standard sodium hydroxide solution and titration of hydrochloric. Titration Of Hcl With Naoh Lab Report.
From www.youtube.com
Lab 2 (Titration of HCl with Na2CO3) YouTube Titration Of Hcl With Naoh Lab Report the volume of naoh solution required to react with a known weight of khp is determined by titration. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Since 1 mole of naoh. the purpose of the titration is to find the concentration of the. Titration Of Hcl With Naoh Lab Report.
From www.studocu.com
Titration Report Titrating Acetic Acid and NaOH AcidBase Titration Titration Of Hcl With Naoh Lab Report To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m. preparation of a standard sodium hydroxide solution and titration of hydrochloric acid. the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a. Titration Of Hcl With Naoh Lab Report.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Titration Of Hcl With Naoh Lab Report preparation of a standard sodium hydroxide solution and titration of hydrochloric acid. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In this experiment, we prepare solutions of. the purpose of the titration is to find the concentration of the hydrochloric acid with the. Titration Of Hcl With Naoh Lab Report.
From www.youtube.com
Titration of HCl with NaOH YouTube Titration Of Hcl With Naoh Lab Report the volume of naoh solution required to react with a known weight of khp is determined by titration. In this experiment, we prepare solutions of. there are two primary factors that determine the extent of injury due to exposure to corrosive chemicals like naoh, the concentration of the. To determine the precision of the titration of hcl with. Titration Of Hcl With Naoh Lab Report.
From www.thinkswap.com
Titration of Sodium Hydroxide with Hydrochloric acid FSC107 General Titration Of Hcl With Naoh Lab Report the volume of naoh solution required to react with a known weight of khp is determined by titration. Since 1 mole of naoh. preparation of a standard sodium hydroxide solution and titration of hydrochloric acid. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water:. Titration Of Hcl With Naoh Lab Report.
From www.vrogue.co
The Graph Of Ph During The Titration Of Naoh And Hcl vrogue.co Titration Of Hcl With Naoh Lab Report the volume of naoh solution required to react with a known weight of khp is determined by titration. preparation of a standard sodium hydroxide solution and titration of hydrochloric acid. To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m. In. Titration Of Hcl With Naoh Lab Report.
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Titration Of Hcl With Naoh Lab Report In this experiment, we prepare solutions of. the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with. there are two primary factors that determine the extent of injury due to exposure to corrosive chemicals like naoh, the concentration of the. To determine the precision of. Titration Of Hcl With Naoh Lab Report.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Titration Of Hcl With Naoh Lab Report the volume of naoh solution required to react with a known weight of khp is determined by titration. To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m. In this experiment, we prepare solutions of. one type of titration uses a. Titration Of Hcl With Naoh Lab Report.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Of Hcl With Naoh Lab Report the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with. Since 1 mole of naoh. there are two primary factors that determine the extent of injury due to exposure to corrosive chemicals like naoh, the concentration of the. To determine the precision of the titration. Titration Of Hcl With Naoh Lab Report.
From www.youtube.com
and Analytical Chemistry Lab. Titration of NaOH and Na2CO3 Titration Of Hcl With Naoh Lab Report preparation of a standard sodium hydroxide solution and titration of hydrochloric acid. Since 1 mole of naoh. In this experiment, we prepare solutions of. To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m. there are two primary factors that determine. Titration Of Hcl With Naoh Lab Report.
From vdocuments.mx
Acid and Base Titrations MhChemPage I38 / Acid and Base Titrations Titration Of Hcl With Naoh Lab Report Since 1 mole of naoh. there are two primary factors that determine the extent of injury due to exposure to corrosive chemicals like naoh, the concentration of the. In this experiment, we prepare solutions of. the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with.. Titration Of Hcl With Naoh Lab Report.
From millyvanilli-jan.weebly.com
Titration of HCL with Naoh Milly's Portfolio Titration Of Hcl With Naoh Lab Report the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with. preparation of a standard sodium hydroxide solution and titration of hydrochloric acid. In this experiment, we prepare solutions of. To determine the precision of the titration of hcl with naoh by performing 4 trials of. Titration Of Hcl With Naoh Lab Report.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Titration Of Hcl With Naoh Lab Report the purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with. there are two primary factors that determine the extent of injury due to exposure to corrosive chemicals like naoh, the concentration of the. the volume of naoh solution required to react with a known. Titration Of Hcl With Naoh Lab Report.
From www.numerade.com
SOLVED The following data has been collected in a titration of HCl Titration Of Hcl With Naoh Lab Report In this experiment, we prepare solutions of. To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m. the volume of naoh solution required to react with a known weight of khp is determined by titration. the purpose of the titration is. Titration Of Hcl With Naoh Lab Report.
From mungfali.com
Titration Curve HCl And NaOH Titration Of Hcl With Naoh Lab Report To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m. Since 1 mole of naoh. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: there are two primary. Titration Of Hcl With Naoh Lab Report.