Pm Indicator In Complexometric Titration . Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Find out the methods, indicators, and applications of this technique. Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal ions. These indicators undergo a definite colour change in. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. Complexometric indicators are those indicators that are used in complexometric titrations. Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable.
from www.brainkart.com
Complexometric indicators are those indicators that are used in complexometric titrations. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal ions. Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Find out the methods, indicators, and applications of this technique. These indicators undergo a definite colour change in. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent.
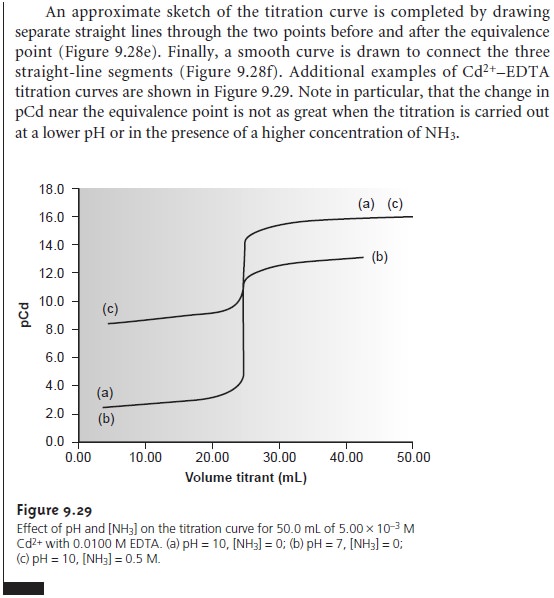
Complexometric EDTA Titration Curves
Pm Indicator In Complexometric Titration These indicators undergo a definite colour change in. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal ions. Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. These indicators undergo a definite colour change in. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Complexometric indicators are those indicators that are used in complexometric titrations. Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Find out the methods, indicators, and applications of this technique.
From www.slideshare.net
Complexometric Titration Pm Indicator In Complexometric Titration Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. These indicators undergo a definite colour change in. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Learn about the chemistry and properties of edta, a multidentate ligand that forms. Pm Indicator In Complexometric Titration.
From www.slideshare.net
Complexometric titrations Pm Indicator In Complexometric Titration Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. Complexometric indicators are those indicators that are used in complexometric titrations. Find out the methods, indicators, and applications of this technique. Learn about the chemistry. Pm Indicator In Complexometric Titration.
From mavink.com
Complexometric Titration Indicator Pm Indicator In Complexometric Titration These indicators undergo a definite colour change in. Complexometric indicators are those indicators that are used in complexometric titrations. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Find out the methods, indicators, and applications of this technique. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation. Pm Indicator In Complexometric Titration.
From www.studypool.com
SOLUTION Complexometric edta titration curves Studypool Pm Indicator In Complexometric Titration Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Learn. Pm Indicator In Complexometric Titration.
From www.slideserve.com
PPT TYBSc Paper IV USCH604 Analytical Chemistry COMPLEXOMETRIC Pm Indicator In Complexometric Titration Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal ions. These indicators undergo a definite colour change. Pm Indicator In Complexometric Titration.
From www.youtube.com
Complexometric titration with EDTAAnimated videoMetal ion indicator Pm Indicator In Complexometric Titration Find out the methods, indicators, and applications of this technique. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal ions. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. Complexometric indicators are those indicators that are used. Pm Indicator In Complexometric Titration.
From www.slideshare.net
Complexometric titration Pm Indicator In Complexometric Titration Complexometric indicators are those indicators that are used in complexometric titrations. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. These indicators undergo a definite colour change in. Find out the methods, indicators, and applications of this technique. Learn how to use complexometric titration to form stable. Pm Indicator In Complexometric Titration.
From www.youtube.com
Part 2 Metal Ion Indicators or pM Indicators for Complexometric Pm Indicator In Complexometric Titration These indicators undergo a definite colour change in. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Complexometric indicators are those indicators that are used in complexometric titrations. Learn how to use complexometric titration to form stable complexes between. Pm Indicator In Complexometric Titration.
From www.youtube.com
Complexometric Titration with EDTAEriochrome BlackTMetal ion Pm Indicator In Complexometric Titration Find out the methods, indicators, and applications of this technique. These indicators undergo a definite colour change in. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal ions. Complexometric titration (sometimes chelatometry) is. Pm Indicator In Complexometric Titration.
From www.studypool.com
SOLUTION Complexometric titration Studypool Pm Indicator In Complexometric Titration Find out the methods, indicators, and applications of this technique. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal ions. Complexometric indicators are those indicators that are used. Pm Indicator In Complexometric Titration.
From brunofuga.adv.br
Complexometric Titrations Types, Indicators, Advantages,, 41 OFF Pm Indicator In Complexometric Titration These indicators undergo a definite colour change in. Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Complexometric indicators are those indicators that are used in complexometric titrations. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Most indicators for complexation. Pm Indicator In Complexometric Titration.
From www.youtube.com
Complexometric titration Factors Different Types Masking Pm Indicator In Complexometric Titration Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Find out the methods, indicators,. Pm Indicator In Complexometric Titration.
From yourindicatinfo.blogspot.com
Complexometric indicator Pm Indicator In Complexometric Titration Find out the methods, indicators, and applications of this technique. Complexometric indicators are those indicators that are used in complexometric titrations. Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. These indicators undergo a definite colour change in. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations. Pm Indicator In Complexometric Titration.
From cetlkybo.blob.core.windows.net
How To Do Complexometric Titration at Thomas Pegg blog Pm Indicator In Complexometric Titration Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Find out the methods, indicators, and applications of this technique. Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used. Pm Indicator In Complexometric Titration.
From collegedunia.com
Complexometric Titration EDTA, Types & Applications Pm Indicator In Complexometric Titration These indicators undergo a definite colour change in. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal ions. Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as. Pm Indicator In Complexometric Titration.
From www.slideshare.net
Complexometric Titration Pm Indicator In Complexometric Titration Complexometric indicators are those indicators that are used in complexometric titrations. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as. Pm Indicator In Complexometric Titration.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration Pm Indicator In Complexometric Titration Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Find out the methods, indicators, and applications of this technique. Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Complexometric indicators are those indicators that are used in complexometric titrations. Complexometric titration. Pm Indicator In Complexometric Titration.
From www.researchgate.net
Indicators for direct complexometric titration of bismuth Download Table Pm Indicator In Complexometric Titration Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. These indicators undergo a definite colour change in. Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent.. Pm Indicator In Complexometric Titration.
From chemistnotes.com
Complexometric Titration Definition, Types, Indicators, and 5 Reliable Pm Indicator In Complexometric Titration Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal ions. Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Complexometric indicators are those indicators that are. Pm Indicator In Complexometric Titration.
From solutionpharmacy.in
Complexometric Titration Solution Parmacy Pm Indicator In Complexometric Titration Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. These indicators undergo a definite colour change in. Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Learn about. Pm Indicator In Complexometric Titration.
From facts.net
17 Intriguing Facts About Complexometric Titration Pm Indicator In Complexometric Titration Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Complexometric indicators are those indicators that are used in complexometric titrations. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable. Pm Indicator In Complexometric Titration.
From slideplayer.com
Complexometric titration Dr.Bhagure G.R. ppt download Pm Indicator In Complexometric Titration Find out the methods, indicators, and applications of this technique. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal ions. These indicators undergo a definite colour change in. Complexometric indicators are those indicators that are used in complexometric titrations. Learn how to use complexometric titration to form stable complexes between. Pm Indicator In Complexometric Titration.
From v-s.mobi
Download Complexometric titration B pharma 1st sem Ph. Analysis Pm Indicator In Complexometric Titration Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Complexometric indicators are those indicators that are used in complexometric. Pm Indicator In Complexometric Titration.
From www.slideshare.net
PAI Complexometric titration.(HRB) PPT Pm Indicator In Complexometric Titration Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Complexometric indicators are those indicators that are used in complexometric titrations. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal ions. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating. Pm Indicator In Complexometric Titration.
From www.brainkart.com
Complexometric EDTA Titration Curves Pm Indicator In Complexometric Titration Complexometric indicators are those indicators that are used in complexometric titrations. These indicators undergo a definite colour change in. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. Most indicators. Pm Indicator In Complexometric Titration.
From www.studypool.com
SOLUTION Complexometric titration note Studypool Pm Indicator In Complexometric Titration Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal ions. Find out the methods, indicators, and applications of this technique. These indicators undergo a definite colour change in. Learn about complex ion formation, stepwise equilibrium reactions, and. Pm Indicator In Complexometric Titration.
From yourindicatinfo.blogspot.com
Complexometric titration Pm Indicator In Complexometric Titration These indicators undergo a definite colour change in. Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Find out the methods, indicators, and applications of this technique. Complexometric indicators are those indicators that are used in complexometric titrations. Learn about the chemistry and properties of edta, a multidentate ligand. Pm Indicator In Complexometric Titration.
From chem.libretexts.org
9.3 Complexation Titrations Chemistry LibreTexts Pm Indicator In Complexometric Titration Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal ions. Find out the methods, indicators, and applications of this technique. Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent.. Pm Indicator In Complexometric Titration.
From www.slideshare.net
Complexometric titration Pm Indicator In Complexometric Titration Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Find out the methods, indicators, and applications of this technique. Complexometric indicators are those indicators that are used in complexometric titrations. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal. Pm Indicator In Complexometric Titration.
From www.chegg.com
Solved The indicator used in the complexometric titration Pm Indicator In Complexometric Titration Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. These indicators undergo a definite colour change in.. Pm Indicator In Complexometric Titration.
From www.chemistrylearner.com
Complexometric Titration Definition, Examples, and Applications Pm Indicator In Complexometric Titration Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Complexometric indicators are those indicators that are used in complexometric titrations. These indicators undergo a definite colour change in. Find out the methods,. Pm Indicator In Complexometric Titration.
From www.studocu.com
Complexometric Titration Pharmaceutical Analysis Studocu Pm Indicator In Complexometric Titration Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Learn about complex ion formation, stepwise equilibrium reactions, and complexometric titrations using edta as a chelating agent. Complexometric indicators are those indicators that are used in complexometric. Pm Indicator In Complexometric Titration.
From chem.libretexts.org
9.3 Complexation Titrations Chemistry LibreTexts Pm Indicator In Complexometric Titration Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal ions. These indicators undergo a definite colour change in. Complexometric indicators are those indicators that are used in complexometric titrations. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to. Pm Indicator In Complexometric Titration.
From pharma2study.blogspot.com
Complexometric titration Pm Indicator In Complexometric Titration Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable. Complexometric indicators are those indicators. Pm Indicator In Complexometric Titration.
From cetlkybo.blob.core.windows.net
How To Do Complexometric Titration at Thomas Pegg blog Pm Indicator In Complexometric Titration Learn how to use complexometric titration to form stable complexes between metal ions and specific complexing agents, such as edta. Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate. These indicators undergo a definite colour change in. Learn about the chemistry and properties of edta, a multidentate. Pm Indicator In Complexometric Titration.