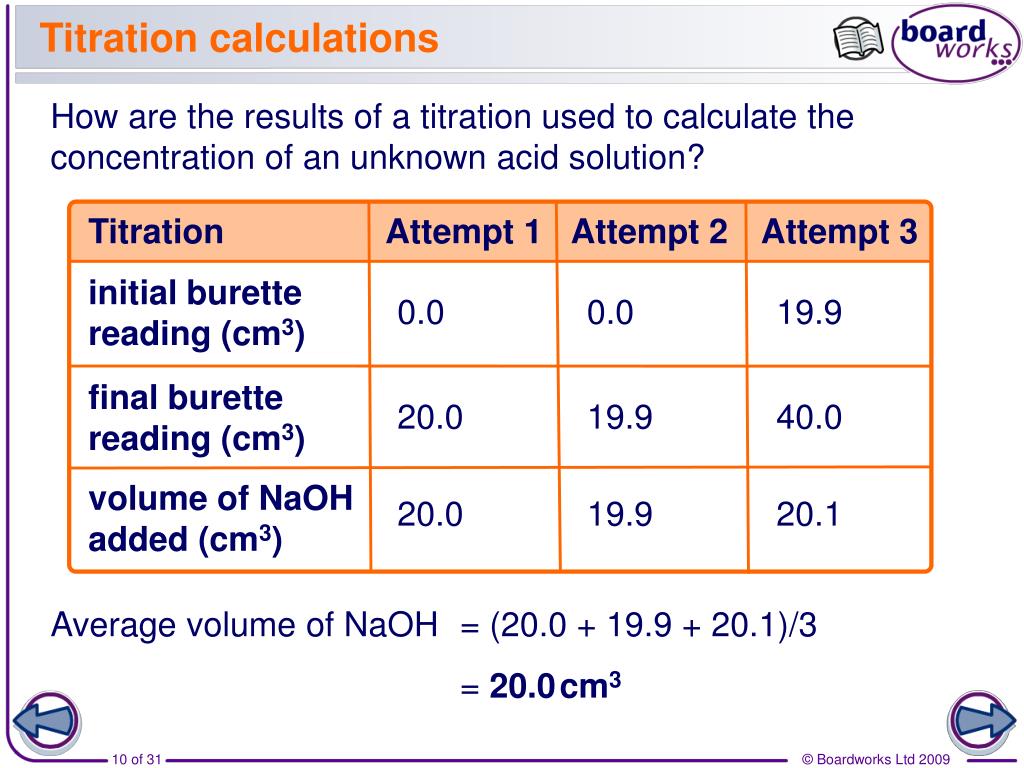
Titration Examples . A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. This video walks through common examples of calculation problems for titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Perform and interpret titration calculations. How to do titration calculations. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A titration experiment aims to determine the concentration of an unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte.
from
How to do titration calculations. A titration experiment aims to determine the concentration of an unknown. Perform and interpret titration calculations. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte.
Titration Examples Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. This video walks through common examples of calculation problems for titration. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. How to do titration calculations. A titration experiment aims to determine the concentration of an unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Perform and interpret titration calculations.
From
Titration Examples Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed,. Titration Examples.
From
Titration Examples Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. How to do titration calculations. A titration experiment aims to determine the concentration of an unknown. This. Titration Examples.
From
Titration Examples A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. A titration experiment aims to determine the concentration of an unknown. This process continues until stoichiometrically equivalent amounts of the reactants. Titration Examples.
From www.slideshare.net
Complexometric titrations Titration Examples The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. How to do titration calculations. The basic process involves adding a standard solution of one reagent to a known amount of. Titration Examples.
From
Titration Examples How to do titration calculations. This video walks through common examples of calculation problems for titration. Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Perform and interpret titration calculations.. Titration Examples.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d Titration Examples This video walks through common examples of calculation problems for titration. Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration involves the gradual addition of a. Titration Examples.
From www.vrogue.co
Strong Acid Weak Base Titrations vrogue.co Titration Examples This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. This video walks through common examples of calculation problems for titration. Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. Titration involves the gradual addition of a. Titration Examples.
From
Titration Examples Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. How to do titration calculations. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Redox titration involves the transfer of electrons. Titration Examples.
From www.science-revision.co.uk
Titrations Titration Examples Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. How to do titration calculations. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A titration experiment aims to determine the concentration of an unknown. Perform and. Titration Examples.
From www.reagent.co.uk
Who Invented Titration? The Science Blog Titration Examples Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. Perform and interpret titration calculations. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The example below. Titration Examples.
From
Titration Examples Perform and interpret titration calculations. Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. This video walks through common examples of calculation problems for titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition. Titration Examples.
From
Titration Examples Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. This video walks through common examples of calculation problems for titration. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. How to do titration calculations. A titration experiment aims to. Titration Examples.
From
Titration Examples Perform and interpret titration calculations. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is the slow. Titration Examples.
From
Titration Examples Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. A titration is a laboratory technique used to precisely measure molar concentration of. Titration Examples.
From lessoncampusphenols.z13.web.core.windows.net
Acid And Base Calculator Titration Examples A titration experiment aims to determine the concentration of an unknown. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. The basic process involves adding a standard solution of one. Titration Examples.
From
Titration Examples Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. A titration experiment aims to determine the concentration of an unknown. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a. Titration Examples.
From
Titration Examples The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. Titration. Titration Examples.
From
Titration Examples This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. This video walks through common examples of calculation problems for titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The example below demonstrates the technique. Titration Examples.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Titration Examples Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. How to do titration calculations. The basic process. Titration Examples.
From www.slideserve.com
PPT Volumetric Analysis PowerPoint Presentation ID439207 Titration Examples The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a. Titration Examples.
From www.scribd.com
Titration PDF Titration Chemistry Titration Examples The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. This video walks through common examples of calculation problems for titration. A titration experiment aims to determine. Titration Examples.
From
Titration Examples A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration experiment aims to determine the concentration of an unknown. This video walks through common examples of calculation problems for titration. Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing. Titration Examples.
From
Titration Examples A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. This video walks through common examples of calculation problems for titration. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. Titration involves the gradual addition of. Titration Examples.
From
Titration Examples This video walks through common examples of calculation problems for titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. This. Titration Examples.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Examples A titration experiment aims to determine the concentration of an unknown. This video walks through common examples of calculation problems for titration. Perform and interpret titration calculations. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A titration is a laboratory technique used to precisely measure molar concentration of. Titration Examples.
From www.sliderbase.com
Titration Titration Examples This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. A titration is a laboratory technique used to precisely. Titration Examples.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 Titration Examples Perform and interpret titration calculations. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. How to do titration calculations. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. This process continues until stoichiometrically equivalent amounts of the reactants. Titration Examples.
From
Titration Examples How to do titration calculations. Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. Titration involves the gradual addition of a reagent of known concentration, known as. Titration Examples.
From
Titration Examples Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. A titration experiment aims to determine the concentration of an unknown. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. Titration Examples.
From
Titration Examples Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. Perform and interpret titration calculations. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. How to do titration calculations. A titration experiment aims to determine the concentration of an unknown.. Titration Examples.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration Examples The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. Perform and interpret titration calculations. A titration experiment aims to determine the concentration of an unknown. Titration. Titration Examples.
From
Titration Examples This video walks through common examples of calculation problems for titration. Perform and interpret titration calculations. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Redox titration involves the transfer of electrons and is used to determine the concentration of oxidizing or reducing agents. A titration is a laboratory. Titration Examples.
From
Titration Examples This video walks through common examples of calculation problems for titration. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. A titration experiment aims to determine the concentration of an unknown. Titration is the slow addition of one solution of a known concentration (called a titrant). Titration Examples.
From
Titration Examples Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A titration experiment aims to determine the concentration of. Titration Examples.
From
Titration Examples This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a standard solution of one reagent to a known amount of the. Titration Examples.