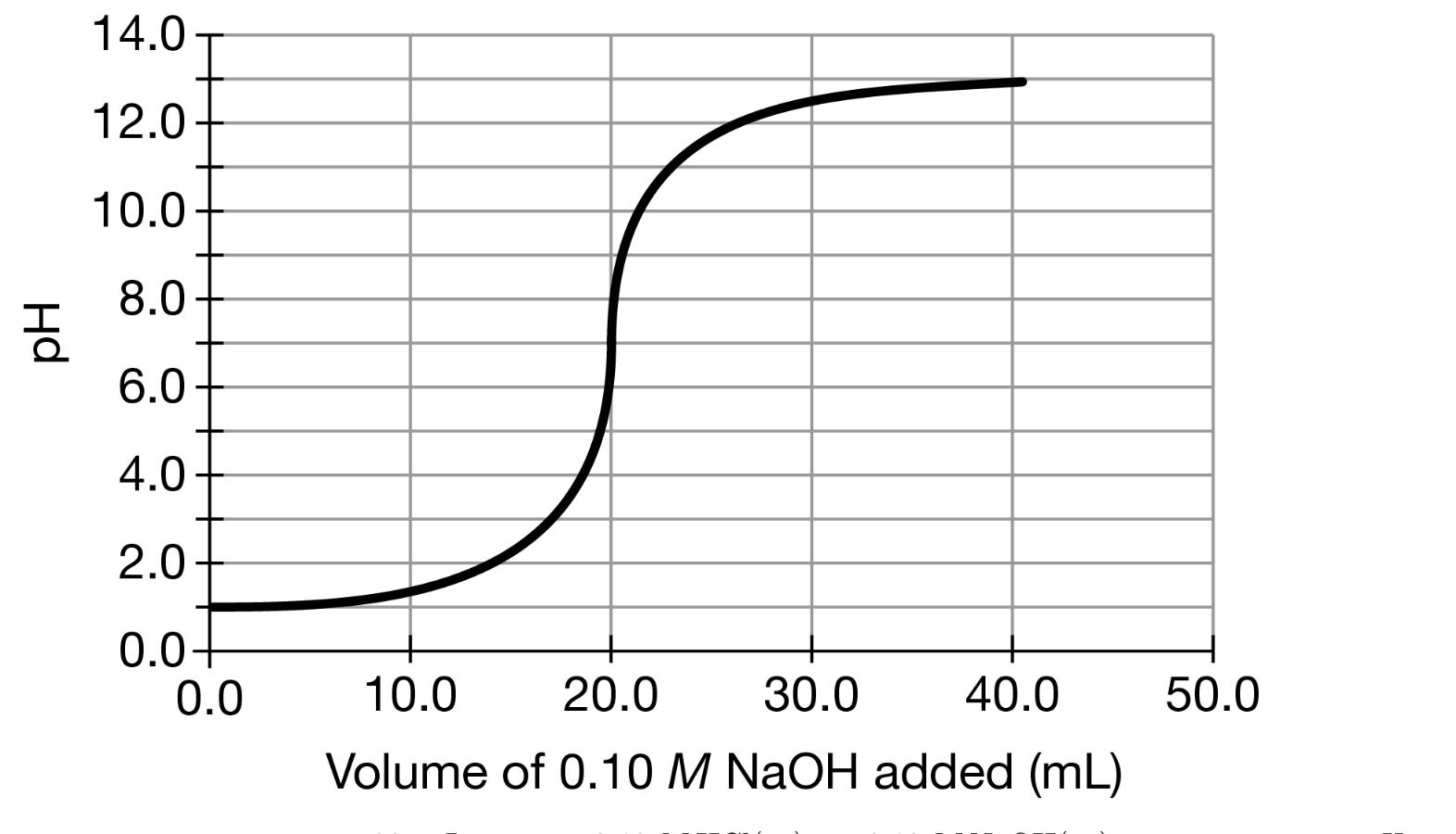
H2So4 And Naoh Titration Curve . Sulfuric acid, h 2 so 4 is a strong diprotic acid. (a) locating the equivalence point volume; Although each titration curve has. This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? (b) plotting two points before the red Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Average the values for the total volumes of naoh added. If a third titration was required, average the two closest values. Sulfuric acid dissociates (ionises) in two stages: each titration curve is for 50.0 ml of 0.0500 m weak acid using 0.100 m naoh. illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: types of titration curves and its features. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,.
from www.vrogue.co
When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. Average the values for the total volumes of naoh added. If a third titration was required, average the two closest values. types of titration curves and its features. Sulfuric acid dissociates (ionises) in two stages: (b) plotting two points before the red This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. each titration curve is for 50.0 ml of 0.0500 m weak acid using 0.100 m naoh. Although each titration curve has.
Solved The Titration Curve Shown Below Is For The Tit vrogue.co
H2So4 And Naoh Titration Curve Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. If a third titration was required, average the two closest values. Sulfuric acid, h 2 so 4 is a strong diprotic acid. Average the values for the total volumes of naoh added. each titration curve is for 50.0 ml of 0.0500 m weak acid using 0.100 m naoh. illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: Sulfuric acid dissociates (ionises) in two stages: (a) locating the equivalence point volume; Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Use the values for the averaged. Although each titration curve has. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? types of titration curves and its features. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,. (b) plotting two points before the red This ensures that the interior portion of the burette is coated with a thin layer of naoh solution.
From www.numerade.com
SOLVED 8 Shown bclow is titration curve for carbonic acid verzus H2So4 And Naoh Titration Curve Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? (b) plotting two points before the red When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,. illustrations showing the steps used to sketch an approximate titration curve for the titration of. H2So4 And Naoh Titration Curve.
From dxouvlqox.blob.core.windows.net
Sulphuric Acid Titration With Sodium Hydroxide at Bernice Kennedy blog H2So4 And Naoh Titration Curve Sulfuric acid, h 2 so 4 is a strong diprotic acid. types of titration curves and its features. Sulfuric acid dissociates (ionises) in two stages: This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4.. H2So4 And Naoh Titration Curve.
From www.chegg.com
Titration of sulfuric acid Average Volume of NaOH = H2So4 And Naoh Titration Curve Sulfuric acid dissociates (ionises) in two stages: Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Sulfuric acid, h 2 so 4 is a strong diprotic acid.. H2So4 And Naoh Titration Curve.
From www.researchgate.net
Plots obtained during titration of 20.00 mL H2SO4 0.024 M + 20.0 mL H2So4 And Naoh Titration Curve Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. types of titration curves and its features. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? (b) plotting two points before the red When titrating a strong. H2So4 And Naoh Titration Curve.
From www.chegg.com
Solved Use the following titration curve for the first four H2So4 And Naoh Titration Curve Sulfuric acid, h 2 so 4 is a strong diprotic acid. each titration curve is for 50.0 ml of 0.0500 m weak acid using 0.100 m naoh. types of titration curves and its features. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,. (b) plotting two points before the red illustrations showing the steps. H2So4 And Naoh Titration Curve.
From www.vrogue.co
Solved The Titration Curve Shown Below Is For The Tit vrogue.co H2So4 And Naoh Titration Curve This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: Sulfuric acid, h 2 so 4 is a strong diprotic acid. (a) locating the. H2So4 And Naoh Titration Curve.
From exogrnoza.blob.core.windows.net
Titration Curve Acetic Acid And Naoh at Isabel Keith blog H2So4 And Naoh Titration Curve Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? Sulfuric acid, h 2 so 4 is a strong diprotic acid. types of titration curves and its. H2So4 And Naoh Titration Curve.
From exoqwdiya.blob.core.windows.net
H2So4 Titration Graph at Jewell Hamilton blog H2So4 And Naoh Titration Curve Average the values for the total volumes of naoh added. types of titration curves and its features. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200. H2So4 And Naoh Titration Curve.
From dxoejubzg.blob.core.windows.net
Titration H2So4 at Gallo blog H2So4 And Naoh Titration Curve Although each titration curve has. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. (b) plotting two points before the red If a third titration was required, average the two closest values. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Average the values for. H2So4 And Naoh Titration Curve.
From www.chegg.com
Solved The following is the titration curve of a 50.0 mL H2So4 And Naoh Titration Curve (a) locating the equivalence point volume; Use the values for the averaged. (b) plotting two points before the red types of titration curves and its features. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of. H2So4 And Naoh Titration Curve.
From mavink.com
Titration Labeled H2So4 And Naoh Titration Curve each titration curve is for 50.0 ml of 0.0500 m weak acid using 0.100 m naoh. Average the values for the total volumes of naoh added. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. Sulfuric acid, h 2 so 4 is a strong diprotic acid. (b) plotting two points before the. H2So4 And Naoh Titration Curve.
From mavink.com
H2so4 Titration Curve H2So4 And Naoh Titration Curve Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. Although each titration curve has. (b) plotting two points before the red Average the values for the total volumes of naoh added. Sulfuric acid, h 2 so 4 is a strong diprotic acid. each titration curve is for 50.0 ml of 0.0500 m. H2So4 And Naoh Titration Curve.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts H2So4 And Naoh Titration Curve This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. (b) plotting two points before the red Sulfuric acid dissociates (ionises) in two stages: When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,. Although each titration curve has. Firstly, clean the burette with distilled water (dw), and then rinse. H2So4 And Naoh Titration Curve.
From www.chegg.com
Solved The curve shows the titration of H2SO3 with NaOH. The H2So4 And Naoh Titration Curve If a third titration was required, average the two closest values. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Sulfuric acid, h 2 so 4 is a strong diprotic acid. Although each titration curve has. (b) plotting two points before the red Use the values for the averaged. Example \(\pageindex{3}\) what. H2So4 And Naoh Titration Curve.
From www.numerade.com
SOLVED Question 6 The graphs labeled (I) through (III) show acidbase H2So4 And Naoh Titration Curve Sulfuric acid dissociates (ionises) in two stages: Average the values for the total volumes of naoh added. (b) plotting two points before the red When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. illustrations showing the steps used to sketch. H2So4 And Naoh Titration Curve.
From dxoejubzg.blob.core.windows.net
Titration H2So4 at Gallo blog H2So4 And Naoh Titration Curve (a) locating the equivalence point volume; types of titration curves and its features. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. Average the values for the total volumes of naoh added. Although each titration. H2So4 And Naoh Titration Curve.
From mariela-kcarroll.blogspot.com
Titration Curve of Hcl and Naoh H2So4 And Naoh Titration Curve Average the values for the total volumes of naoh added. Sulfuric acid dissociates (ionises) in two stages: Sulfuric acid, h 2 so 4 is a strong diprotic acid. each titration curve is for 50.0 ml of 0.0500 m weak acid using 0.100 m naoh. This ensures that the interior portion of the burette is coated with a thin layer. H2So4 And Naoh Titration Curve.
From exozdjwai.blob.core.windows.net
What Does A Titration Curve Tell Us at Gladys Abreu blog H2So4 And Naoh Titration Curve (a) locating the equivalence point volume; When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,. Although each titration curve has. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Average the values for the total volumes of naoh added. Sulfuric acid dissociates (ionises) in two stages: each titration. H2So4 And Naoh Titration Curve.
From www.chegg.com
Solved h 7. The graph below is the titration curve of a 100 H2So4 And Naoh Titration Curve types of titration curves and its features. This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. each titration curve is for 50.0 ml of 0.0500 m weak acid using 0.100 m naoh. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution.. H2So4 And Naoh Titration Curve.
From mavink.com
H2so4 Titration Curve H2So4 And Naoh Titration Curve (b) plotting two points before the red Although each titration curve has. If a third titration was required, average the two closest values. illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: Average the values for the total volumes of naoh. H2So4 And Naoh Titration Curve.
From www.chegg.com
Solved 6B Experiment Acid Base titration of H2SO4 and NaOH. H2So4 And Naoh Titration Curve Sulfuric acid dissociates (ionises) in two stages: This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,. Average the values for the total volumes of naoh added. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh. H2So4 And Naoh Titration Curve.
From www.researchgate.net
Titration of 1 mL diluted bath (H2SO4/H3PO4) with 0.5 M NaOH in water H2So4 And Naoh Titration Curve Sulfuric acid, h 2 so 4 is a strong diprotic acid. types of titration curves and its features. (b) plotting two points before the red Use the values for the averaged. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. Sulfuric acid dissociates (ionises) in two stages: Average the values for the. H2So4 And Naoh Titration Curve.
From mavink.com
H2so4 Titration Curve H2So4 And Naoh Titration Curve When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Use the values for the averaged. (a) locating the equivalence point volume; Although each titration curve has. each titration curve is for 50.0 ml of 0.0500 m weak acid using. H2So4 And Naoh Titration Curve.
From www.chegg.com
Solved HCl NaOH Titration Curve CH3COOH NaOH Titration H2So4 And Naoh Titration Curve (a) locating the equivalence point volume; Use the values for the averaged. types of titration curves and its features. illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: each titration curve is for 50.0 ml of 0.0500 m weak. H2So4 And Naoh Titration Curve.
From allisson-blogweber.blogspot.com
H2so4 Naoh Balanced Equation H2So4 And Naoh Titration Curve Sulfuric acid, h 2 so 4 is a strong diprotic acid. This ensures that the interior portion of the burette is coated with a thin layer of naoh solution. Although each titration curve has. types of titration curves and its features. each titration curve is for 50.0 ml of 0.0500 m weak acid using 0.100 m naoh. Sulfuric. H2So4 And Naoh Titration Curve.
From www.numerade.com
SOLVED Draw a titration curve for H2SO4 and NaOH titration. Label and H2So4 And Naoh Titration Curve Sulfuric acid, h 2 so 4 is a strong diprotic acid. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? types of titration curves and its features. If a third titration was required, average the two closest values. Average the values for the. H2So4 And Naoh Titration Curve.
From mungfali.com
Sulfuric Acid Titration Curve H2So4 And Naoh Titration Curve Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Average the values for the total volumes of naoh added. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,. Sulfuric acid, h 2 so 4 is a strong diprotic acid. Although each titration curve has. Example \(\pageindex{3}\) what is the. H2So4 And Naoh Titration Curve.
From byjus.com
The graph of pH during the titration of NaOH and HCl H2So4 And Naoh Titration Curve Although each titration curve has. Average the values for the total volumes of naoh added. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. (a) locating the equivalence point volume; types of titration curves and its features. each titration curve is for 50.0 ml of 0.0500 m weak acid using. H2So4 And Naoh Titration Curve.
From www.chegg.com
Titration Curve H2SO4/NaOH At each point in the H2So4 And Naoh Titration Curve Although each titration curve has. each titration curve is for 50.0 ml of 0.0500 m weak acid using 0.100 m naoh. (b) plotting two points before the red Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,. Clean the. H2So4 And Naoh Titration Curve.
From www.numerade.com
SOLVED Question 16 1 Volume of Acid (mL) Examine the titration curve H2So4 And Naoh Titration Curve Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. Sulfuric acid dissociates (ionises) in two stages: illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: When titrating a strong acid, such as hydrochloric acid. H2So4 And Naoh Titration Curve.
From www.chegg.com
A titration curve is shown below for a solution of H2So4 And Naoh Titration Curve Use the values for the averaged. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? (a) locating the equivalence point volume; (b) plotting two points before the red each titration curve. H2So4 And Naoh Titration Curve.
From exolnazbp.blob.core.windows.net
A Titration Reached The Equivalence Point When 16.1 Ml at Amber Holmes blog H2So4 And Naoh Titration Curve Although each titration curve has. Sulfuric acid, h 2 so 4 is a strong diprotic acid. Sulfuric acid dissociates (ionises) in two stages: Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. (b) plotting two points. H2So4 And Naoh Titration Curve.
From psu.pb.unizin.org
AcidBase Titrations (14.7) Chemistry 112 (Chapters 1217 of OpenStax H2So4 And Naoh Titration Curve Average the values for the total volumes of naoh added. Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. (a) locating the equivalence point volume; Clean the pipette with dw, and rinse it with dilute solution of h 2 so 4. This ensures that the interior portion of the burette is coated. H2So4 And Naoh Titration Curve.
From mavink.com
H2so4 Titration Curve H2So4 And Naoh Titration Curve each titration curve is for 50.0 ml of 0.0500 m weak acid using 0.100 m naoh. (a) locating the equivalence point volume; Firstly, clean the burette with distilled water (dw), and then rinse it with sodium hydroxide (naoh) solution. Average the values for the total volumes of naoh added. (b) plotting two points before the red Clean the pipette. H2So4 And Naoh Titration Curve.
From dxonsvczs.blob.core.windows.net
Titration Base Concentration at James Cummings blog H2So4 And Naoh Titration Curve When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,. types of titration curves and its features. illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: Average the values for the total volumes of naoh added. (a) locating. H2So4 And Naoh Titration Curve.