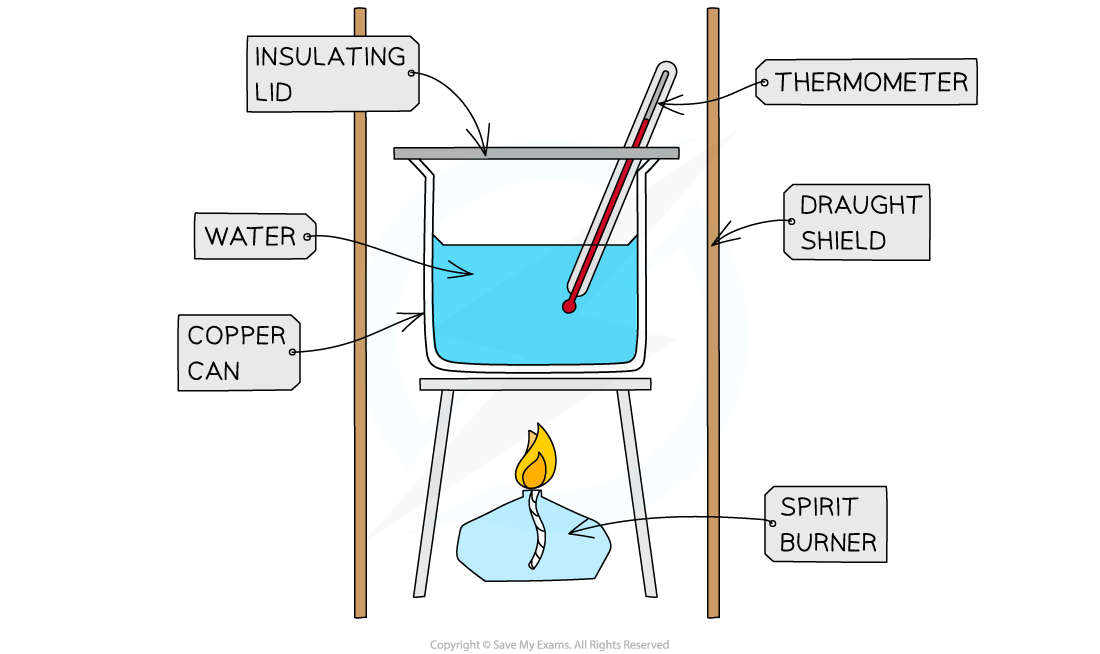
Calorimetry Chemistry Experiment . calorimetry is the science of measuring heat flow. measuring heat transfers is called calorimetry. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. lab session 9, experiment 8: Heat is defined as thermal energy flowing from an object at a higher temperature to one. calorimetry is used to physically measure changes in enthalpy. The diagram shows a simple calorimetry experiment to measure the. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that.
from exozspczc.blob.core.windows.net
calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the science of measuring heat flow. Heat is defined as thermal energy flowing from an object at a higher temperature to one. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. calorimetry is used to physically measure changes in enthalpy. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy. lab session 9, experiment 8:
Calorimeter Experiment Introduction at Jose Evans blog
Calorimetry Chemistry Experiment Heat is defined as thermal energy flowing from an object at a higher temperature to one. the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. calorimetry is the science of measuring heat flow. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. lab session 9, experiment 8: The diagram shows a simple calorimetry experiment to measure the. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Heat is defined as thermal energy flowing from an object at a higher temperature to one. measuring heat transfers is called calorimetry. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. calorimetry is used to physically measure changes in enthalpy.
From exohkbfgq.blob.core.windows.net
Calorimeter For Experiment at Lillian Bordner blog Calorimetry Chemistry Experiment Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Heat is defined as thermal energy flowing from an object at a higher temperature to one. calorimetry is used to physically measure changes in enthalpy. in this experiment you will heat a known mass of a metal to a known temperature. Calorimetry Chemistry Experiment.
From printablelistprodded.z21.web.core.windows.net
Specific Heat Calculation Examples Calorimetry Chemistry Experiment calorimetry is the science of measuring heat flow. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. The diagram shows a simple calorimetry experiment to measure the. lab session 9, experiment 8: calorimetry is the measurement of the transfer of heat into or out of a system during a. Calorimetry Chemistry Experiment.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Chemistry Experiment The diagram shows a simple calorimetry experiment to measure the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Specific heat is an intensive. Calorimetry Chemistry Experiment.
From www.bluesandstemlabs.com
Virtual Stem Lab BlueSands STEM Labs Calorimetry Chemistry Experiment a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the science of measuring heat flow. calorimetry is used to physically measure changes in enthalpy. the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. The. Calorimetry Chemistry Experiment.
From www.linkedin.com
Sara Drvarič Talian on LinkedIn battery batteryresearch Calorimetry Chemistry Experiment in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. measuring heat transfers is called calorimetry. calorimetry is the science of measuring heat flow. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Calorimetry Chemistry Experiment.
From www.studocu.com
P calorimetry 25 lab report StuDocu Calorimetry Chemistry Experiment Heat is defined as thermal energy flowing from an object at a higher temperature to one. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the science of measuring heat flow. calorimetry is used to physically measure changes in enthalpy. the. Calorimetry Chemistry Experiment.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.2 Describe Simple Calorimetry Experiments for Calorimetry Chemistry Experiment calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. calorimetry is used to physically measure changes. Calorimetry Chemistry Experiment.
From www.bluesandstemlabs.com
Virtual Stem Lab BlueSands STEM Labs Calorimetry Chemistry Experiment calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Heat is defined as thermal energy flowing from an object at a higher temperature to one. the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. . Calorimetry Chemistry Experiment.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Chemistry Experiment a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. lab session 9, experiment 8: calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. The diagram shows a simple calorimetry experiment to measure the. . Calorimetry Chemistry Experiment.
From schoolbag.info
Figure 7.6. Diagram of a Bomb Calorimeter Calorimetry Chemistry Experiment in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. calorimetry is the science of measuring heat flow. Heat is defined as thermal energy flowing from an object at a higher temperature to one. lab session 9, experiment 8: calorimetry is used. Calorimetry Chemistry Experiment.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimetry Chemistry Experiment the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Heat is defined. Calorimetry Chemistry Experiment.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calorimetry Chemistry Experiment calorimetry is the science of measuring heat flow. calorimetry is used to physically measure changes in enthalpy. The diagram shows a simple calorimetry experiment to measure the. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. the amount of heat energy released by a chemical reaction can be measured. Calorimetry Chemistry Experiment.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Calorimetry Chemistry Experiment the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. calorimetry is the science of measuring heat flow. measuring heat transfers is called calorimetry. calorimetry is used to physically measure. Calorimetry Chemistry Experiment.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter Calorimetry Chemistry Experiment Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy. lab session 9, experiment 8: in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. measuring heat transfers is called calorimetry. the amount of heat energy released by a chemical. Calorimetry Chemistry Experiment.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts Calorimetry Chemistry Experiment a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. lab session 9, experiment 8: measuring heat transfers is called calorimetry. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. The diagram shows a. Calorimetry Chemistry Experiment.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Chemistry Experiment Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy. calorimetry is the science of measuring heat flow. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. lab session 9, experiment 8: a calorimeter is a device used to measure the. Calorimetry Chemistry Experiment.
From exozspczc.blob.core.windows.net
Calorimeter Experiment Introduction at Jose Evans blog Calorimetry Chemistry Experiment in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. lab session 9, experiment 8: The diagram shows a simple calorimetry experiment to measure the. the. Calorimetry Chemistry Experiment.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimetry Chemistry Experiment a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. measuring heat transfers is called calorimetry. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it. Calorimetry Chemistry Experiment.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Chemistry Experiment Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Heat is defined as thermal energy flowing from an object at a higher temperature to one. lab. Calorimetry Chemistry Experiment.
From www.youtube.com
Heat of Reaction (Calorimetry) Experiment YouTube Calorimetry Chemistry Experiment The diagram shows a simple calorimetry experiment to measure the. calorimetry is the science of measuring heat flow. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. measuring heat transfers is called calorimetry. lab session 9, experiment 8: Heat is defined as thermal energy flowing from an object at. Calorimetry Chemistry Experiment.
From www.studocu.com
Experiment6 Chemistry experiment for Calorimetry Experiment No. 6 Calorimetry Chemistry Experiment lab session 9, experiment 8: measuring heat transfers is called calorimetry. calorimetry is used to physically measure changes in enthalpy. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Specific heat is an intensive property of a single phase (solid, liquid. Calorimetry Chemistry Experiment.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Chemistry Experiment measuring heat transfers is called calorimetry. calorimetry is the science of measuring heat flow. the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. The diagram shows a simple calorimetry experiment to measure the. calorimetry is used to physically measure changes in enthalpy. Bomb calorimetry uses a. Calorimetry Chemistry Experiment.
From www.youtube.com
Calorimetry Experiment YouTube Calorimetry Chemistry Experiment The diagram shows a simple calorimetry experiment to measure the. calorimetry is used to physically measure changes in enthalpy. the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. measuring heat transfers is called calorimetry. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy. . Calorimetry Chemistry Experiment.
From printablelistquinta.z21.web.core.windows.net
Heat Of Formation Equations Calorimetry Chemistry Experiment The diagram shows a simple calorimetry experiment to measure the. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the science of measuring heat flow. calorimetry is used to physically measure changes in enthalpy. in this experiment you will heat a. Calorimetry Chemistry Experiment.
From www.youtube.com
Calorimetry calculation YouTube Calorimetry Chemistry Experiment the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. measuring heat transfers is called calorimetry. calorimetry is the science of measuring heat flow. The diagram shows a simple calorimetry experiment to measure the. calorimetry is used to physically measure changes in enthalpy. lab session 9,. Calorimetry Chemistry Experiment.
From cerifsbe.blob.core.windows.net
Calorimetry Fuels Experiment at Francis Edler blog Calorimetry Chemistry Experiment calorimetry is the science of measuring heat flow. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy. Heat is defined as thermal energy flowing from an object at a higher temperature to one. measuring heat transfers is called calorimetry. the amount of heat energy released by a chemical reaction can be measured using a. Calorimetry Chemistry Experiment.
From www.studocu.com
Lab Report 6765 labs LAB REPORT Experiment No Name Course Code EXP Calorimetry Chemistry Experiment calorimetry is used to physically measure changes in enthalpy. The diagram shows a simple calorimetry experiment to measure the. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample. Calorimetry Chemistry Experiment.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Chemistry Experiment calorimetry is used to physically measure changes in enthalpy. lab session 9, experiment 8: measuring heat transfers is called calorimetry. Heat is defined as thermal energy flowing from an object at a higher temperature to one. calorimetry is the science of measuring heat flow. The diagram shows a simple calorimetry experiment to measure the. a. Calorimetry Chemistry Experiment.
From chem.libretexts.org
6.3 Calorimetry Chemistry LibreTexts Calorimetry Chemistry Experiment the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. lab session 9, experiment 8: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. calorimetry is the measurement of the transfer of heat into or out of a system during. Calorimetry Chemistry Experiment.
From www.studocu.com
At Home Molecular Shapes General Chemistry I Laboratory Experiment At Calorimetry Chemistry Experiment The diagram shows a simple calorimetry experiment to measure the. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Heat is defined as thermal energy flowing from an object at a higher temperature to one. a calorimeter is a device used to measure. Calorimetry Chemistry Experiment.
From www.studocu.com
MCS preprint 1 2019 0219 HS PREPRINT AUTHOR ACCEPTED MANUSCRIPT Calorimetry Chemistry Experiment Heat is defined as thermal energy flowing from an object at a higher temperature to one. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. lab session 9, experiment 8: measuring heat transfers is called calorimetry. the amount of heat energy released by. Calorimetry Chemistry Experiment.
From www.studocu.com
Calorimetry (Experiment 3) Laboratory Report Calorimetry (Experiment Calorimetry Chemistry Experiment The diagram shows a simple calorimetry experiment to measure the. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Heat is defined as thermal. Calorimetry Chemistry Experiment.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Chemistry Experiment Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. measuring heat transfers is called calorimetry. Heat is defined as thermal energy flowing from an object at a higher temperature to one. in this experiment you will heat a known mass of a metal to a known temperature and then transfer. Calorimetry Chemistry Experiment.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimetry Chemistry Experiment Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process.. Calorimetry Chemistry Experiment.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Chemistry Experiment calorimetry is used to physically measure changes in enthalpy. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Heat. Calorimetry Chemistry Experiment.