Atomic Radius Of Sulphur And Chlorine . The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. It covers ionisation energy, atomic radius, electronegativity,. Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. This page describes and explains the trends in atomic and physical properties of the period 3 elements from sodium to argon. Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). Atomic radii can be obtained from quantum. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a.
from www.youtube.com
Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. This page describes and explains the trends in atomic and physical properties of the period 3 elements from sodium to argon. It covers ionisation energy, atomic radius, electronegativity,. Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. Atomic radii can be obtained from quantum.
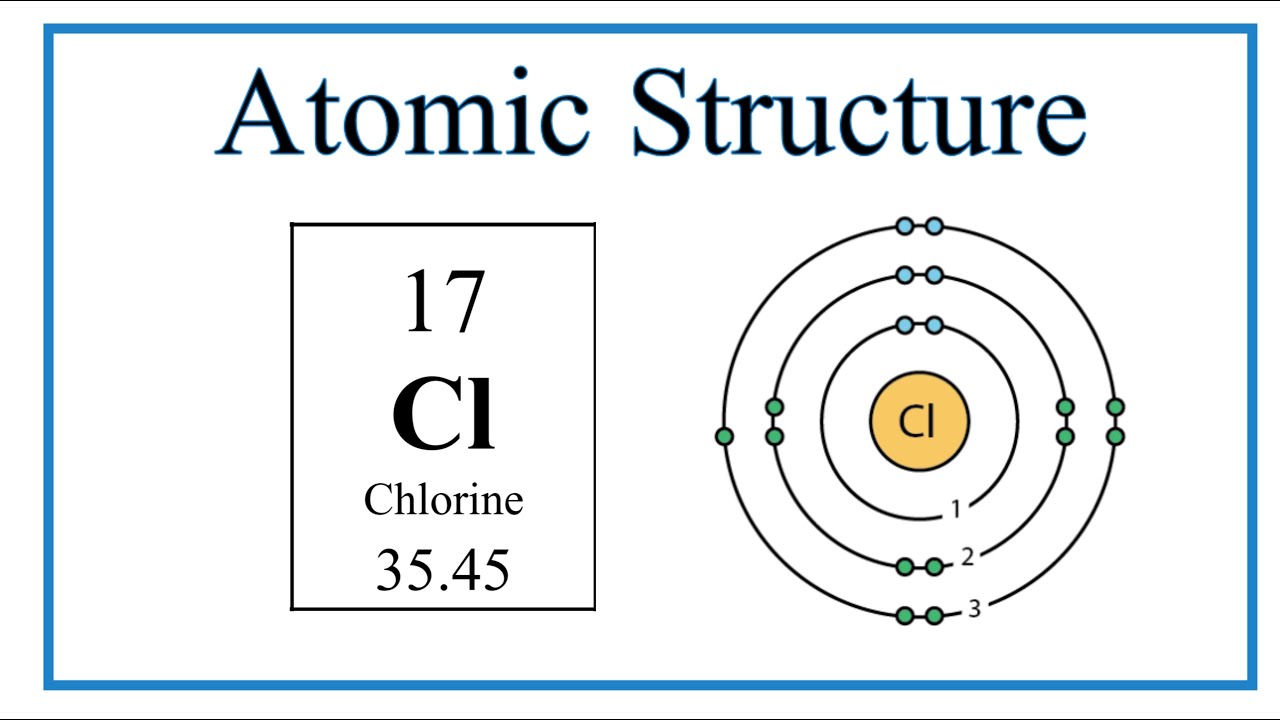
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube
Atomic Radius Of Sulphur And Chlorine Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). It covers ionisation energy, atomic radius, electronegativity,. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. This page describes and explains the trends in atomic and physical properties of the period 3 elements from sodium to argon. Atomic radii can be obtained from quantum. Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a.
From chemistry.stackexchange.com
What is the atomic radius of chlorine? Chemistry Stack Exchange Atomic Radius Of Sulphur And Chlorine It covers ionisation energy, atomic radius, electronegativity,. Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. Atomic radii can be obtained from quantum. Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). The type of atomic. Atomic Radius Of Sulphur And Chlorine.
From www.coursehero.com
[Solved] 1. Explain why sulfur has a larger atomic radius than chlorine Atomic Radius Of Sulphur And Chlorine It covers ionisation energy, atomic radius, electronegativity,. Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. Sulphur. Atomic Radius Of Sulphur And Chlorine.
From slideplayer.com
Atomic Radius Ionization Energy Electronegativity Reactivity ppt download Atomic Radius Of Sulphur And Chlorine We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. Atomic radii can be obtained from quantum. It covers ionisation energy, atomic radius, electronegativity,. The type of atomic radius being measured here is called the metallic radius or. Atomic Radius Of Sulphur And Chlorine.
From pressbooks.bccampus.ca
1.6 Periodic Variations in Element Properties Chemistry for Atomic Radius Of Sulphur And Chlorine This page describes and explains the trends in atomic and physical properties of the period 3 elements from sodium to argon. Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). Atomic radii can be obtained from quantum. Sulphur also exists as a number of allotropes but the most common. Atomic Radius Of Sulphur And Chlorine.
From chamotgallery.com
Understanding Atomic Radius Trends The 2 Key Principles (2023) Atomic Radius Of Sulphur And Chlorine Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. Atomic radii can be obtained from quantum. Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). It covers ionisation energy, atomic radius, electronegativity,. We assign half of. Atomic Radius Of Sulphur And Chlorine.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Atomic Radius Of Sulphur And Chlorine Atomic radii can be obtained from quantum. It covers ionisation energy, atomic radius, electronegativity,. Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. This page describes and explains. Atomic Radius Of Sulphur And Chlorine.
From www.chem.fsu.edu
Electron Configurations Atomic Radius Of Sulphur And Chlorine Atomic radii can be obtained from quantum. It covers ionisation energy, atomic radius, electronegativity,. This page describes and explains the trends in atomic and physical properties of the period 3 elements from sodium to argon. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei. Atomic Radius Of Sulphur And Chlorine.
From utedzz.blogspot.com
Largest Atomic Radius Periodic Table Periodic Table Timeline Atomic Radius Of Sulphur And Chlorine Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft,. Atomic Radius Of Sulphur And Chlorine.
From sciencenotes.org
Atomic Radius and Ionic Radius Atomic Radius Of Sulphur And Chlorine Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. It covers ionisation energy, atomic radius, electronegativity,. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. This page describes and explains the trends in atomic and. Atomic Radius Of Sulphur And Chlorine.
From material-properties.org
Sulfur and Chlorine Comparison Properties Material Properties Atomic Radius Of Sulphur And Chlorine Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. Sulphur also exists as a number of allotropes. Atomic Radius Of Sulphur And Chlorine.
From gia-kharmon.blogspot.com
What Is the Atomic Radius of Chlorine Atomic Radius Of Sulphur And Chlorine The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. It covers ionisation energy, atomic radius, electronegativity,. Atomic radii can be obtained from quantum. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei. Atomic Radius Of Sulphur And Chlorine.
From www.gauthmath.com
Which element will have the smallest atomic radius? Sulfur (S) Fluorine Atomic Radius Of Sulphur And Chlorine Atomic radii can be obtained from quantum. Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. We assign half of this distance to each chlorine atom,. Atomic Radius Of Sulphur And Chlorine.
From chem.libretexts.org
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic Atomic Radius Of Sulphur And Chlorine Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. It covers ionisation energy, atomic radius, electronegativity,. Description and explanation of the trend in atomic radius going. Atomic Radius Of Sulphur And Chlorine.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Periodic Table Element Comparison Compare Sulfur vs Chlorine Atomic Radius Of Sulphur And Chlorine This page describes and explains the trends in atomic and physical properties of the period 3 elements from sodium to argon. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. Sulphur also exists as a number of. Atomic Radius Of Sulphur And Chlorine.
From www.webelements.com
Elements Periodic Table » Sulfur » properties of free atoms Atomic Radius Of Sulphur And Chlorine It covers ionisation energy, atomic radius, electronegativity,. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline. Atomic Radius Of Sulphur And Chlorine.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision Atomic Radius Of Sulphur And Chlorine Atomic radii can be obtained from quantum. Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. This. Atomic Radius Of Sulphur And Chlorine.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Atomic Radius Of Sulphur And Chlorine Atomic radii can be obtained from quantum. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). Sulphur. Atomic Radius Of Sulphur And Chlorine.
From sciencenotes.org
Periodic Table of Element Atom Sizes Atomic Radius Of Sulphur And Chlorine The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. It covers ionisation energy, atomic radius, electronegativity,. This page describes and explains the trends in atomic and physical properties of the period 3 elements from sodium to argon. Atomic radii can be obtained from quantum. We assign half of. Atomic Radius Of Sulphur And Chlorine.
From wirelistetiquette.z13.web.core.windows.net
Diagram Of Sulfur Atomic Radius Of Sulphur And Chlorine The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. Sulphur also exists as a number of. Atomic Radius Of Sulphur And Chlorine.
From www.shutterstock.com
Types Atomic Radius Chemical Element Nitrogen เวกเตอร์สต็อก (ปลอดค่า Atomic Radius Of Sulphur And Chlorine Atomic radii can be obtained from quantum. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding.. Atomic Radius Of Sulphur And Chlorine.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Atomic Radius Of Sulphur And Chlorine It covers ionisation energy, atomic radius, electronegativity,. This page describes and explains the trends in atomic and physical properties of the period 3 elements from sodium to argon. Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. We assign half of this distance to each chlorine atom,. Atomic Radius Of Sulphur And Chlorine.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Atomic Radius Of Sulphur And Chlorine Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. It covers ionisation energy, atomic radius, electronegativity,. Atomic radii can be obtained from quantum. This page describes and explains. Atomic Radius Of Sulphur And Chlorine.
From sciencenotes.org
Sulfur Atom Science Notes and Projects Atomic Radius Of Sulphur And Chlorine It covers ionisation energy, atomic radius, electronegativity,. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. Atomic radii can be obtained from quantum. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei. Atomic Radius Of Sulphur And Chlorine.
From brainly.com
Arrange these elements in order of decreasing atomic size sulfur Atomic Radius Of Sulphur And Chlorine Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. Description and explanation of the trend. Atomic Radius Of Sulphur And Chlorine.
From neetlab.com
Atomic Radius Periodic Table NEET Lab Atomic Radius Of Sulphur And Chlorine The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. This page describes and explains the trends in atomic and physical properties of the period 3 elements from sodium to argon. It covers ionisation energy, atomic radius, electronegativity,. Description and explanation of the trend in atomic radius going across. Atomic Radius Of Sulphur And Chlorine.
From www.pinterest.co.uk
Sulfur, atomic structure Stock Image C018/3697 Science Photo Atomic Radius Of Sulphur And Chlorine It covers ionisation energy, atomic radius, electronegativity,. Atomic radii can be obtained from quantum. Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. Sulphur also exists as a. Atomic Radius Of Sulphur And Chlorine.
From www.numerade.com
SOLVED Compared to the atomic radius of a sulfur atom. the atomic Atomic Radius Of Sulphur And Chlorine We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. It covers ionisation energy, atomic radius, electronegativity,. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding.. Atomic Radius Of Sulphur And Chlorine.
From www.numerade.com
SOLVED Using only the periodic table arrange the following elements in Atomic Radius Of Sulphur And Chlorine Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). The type of atomic radius being measured here is called the metallic radius or the covalent radius depending. Atomic Radius Of Sulphur And Chlorine.
From courses.lumenlearning.com
Ionic Radius Introduction to Chemistry Atomic Radius Of Sulphur And Chlorine The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. It covers ionisation energy, atomic radius, electronegativity,. Atomic radii can be obtained from quantum. Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. This page describes. Atomic Radius Of Sulphur And Chlorine.
From www.shutterstock.com
Types Atomic Radius Chemical Element Atomic Stock Vector (Royalty Free Atomic Radius Of Sulphur And Chlorine It covers ionisation energy, atomic radius, electronegativity,. Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. This page describes and explains the trends in atomic and physical properties of the period 3 elements from sodium to argon. The type of atomic radius being measured here is called. Atomic Radius Of Sulphur And Chlorine.
From www.vrogue.co
Periodic Table Of The Elements Atomic Radius vrogue.co Atomic Radius Of Sulphur And Chlorine Atomic radii can be obtained from quantum. Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by. Atomic Radius Of Sulphur And Chlorine.
From 88guru.com
Atomic RadiusAn Overview 88Guru Atomic Radius Of Sulphur And Chlorine We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. It covers ionisation energy, atomic radius, electronegativity,.. Atomic Radius Of Sulphur And Chlorine.
From smartquizbasket.blogspot.com
Smart Quiz Basket Atomic Radius Of Sulfur Atomic Radius Of Sulphur And Chlorine The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. It covers ionisation energy, atomic radius, electronegativity,. We assign half of this distance to each chlorine atom,. Atomic Radius Of Sulphur And Chlorine.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure Atomic Radius Of Sulphur And Chlorine Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. It covers ionisation energy, atomic radius,. Atomic Radius Of Sulphur And Chlorine.
From material-properties.org
Sulfur Periodic Table and Atomic Properties Atomic Radius Of Sulphur And Chlorine It covers ionisation energy, atomic radius, electronegativity,. Description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). Sulphur also exists as a number of allotropes but the most common is rhombic sulphur which is a soft, yellow, crystalline solid. Atomic radii can be obtained from quantum. This page describes and. Atomic Radius Of Sulphur And Chlorine.