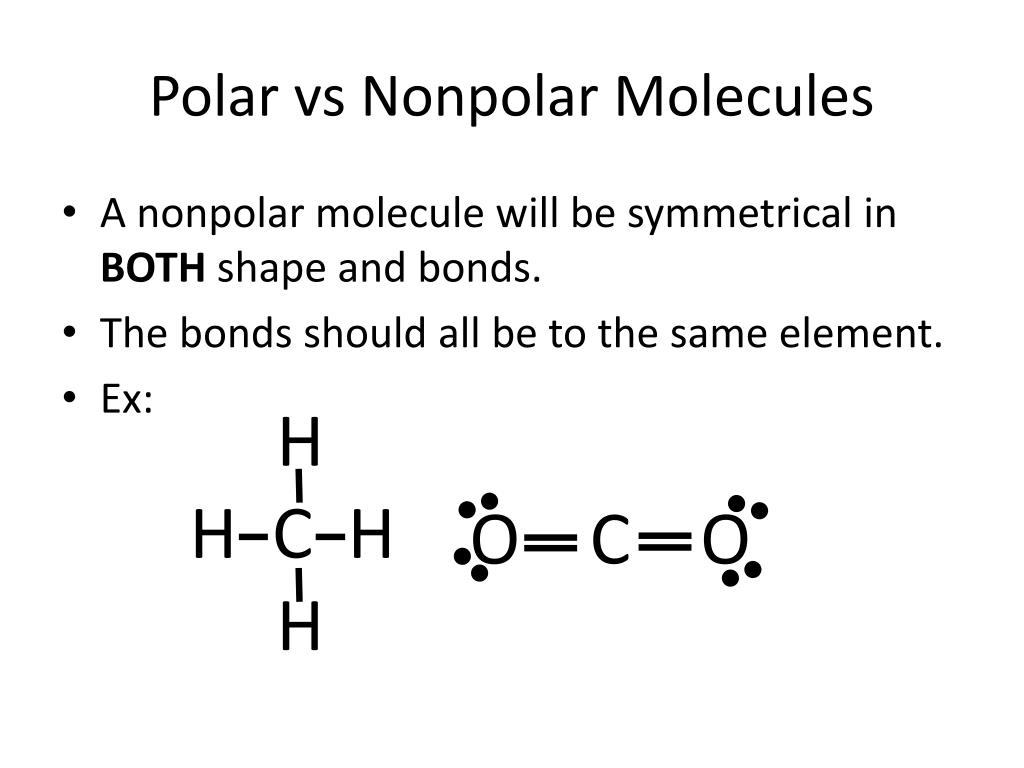
What Bonds Are Non Polar . What is a nonpolar covalent bond? A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent. When the electronegativity difference is. A covalent bond that has an equal sharing of. A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. Both of the bonds inside the molecule are polar bonds. Intermolecular bonds are found between molecules. They are also known as van der waals forces, and there are several types to consider. A covalent bond is formed when two atoms share electrons between them. In a nonpolar covalent bond, electrons are equally shared.
from www.slideserve.com
Both of the bonds inside the molecule are polar bonds. In a nonpolar covalent bond, electrons are equally shared. A covalent bond is formed when two atoms share electrons between them. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent. What is a nonpolar covalent bond? A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. A covalent bond that has an equal sharing of. They are also known as van der waals forces, and there are several types to consider. Intermolecular bonds are found between molecules.
PPT Polar or Nonpolar PowerPoint Presentation, free download ID3483667
What Bonds Are Non Polar A covalent bond that has an equal sharing of. When the electronegativity difference is. A covalent bond is formed when two atoms share electrons between them. A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. Intermolecular bonds are found between molecules. In a nonpolar covalent bond, electrons are equally shared. Both of the bonds inside the molecule are polar bonds. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent. A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. A covalent bond that has an equal sharing of. They are also known as van der waals forces, and there are several types to consider. What is a nonpolar covalent bond?
From www.expii.com
Polar vs. Nonpolar Bonds — Overview & Examples Expii What Bonds Are Non Polar Both of the bonds inside the molecule are polar bonds. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent. A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. When the electronegativity difference is. They are also. What Bonds Are Non Polar.
From surfguppy.com
What is Nonpolar Covalent Bond What Bonds Are Non Polar They are also known as van der waals forces, and there are several types to consider. Both of the bonds inside the molecule are polar bonds. A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. A covalent bond that has an equal sharing of.. What Bonds Are Non Polar.
From www.slideserve.com
PPT Nonpolar Covalent Bonds PowerPoint Presentation, free download What Bonds Are Non Polar They are also known as van der waals forces, and there are several types to consider. A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. Both of the bonds inside the molecule are polar bonds. In a diatomic molecule with two identical atoms, there is no difference in electronegativity,. What Bonds Are Non Polar.
From slideplayer.com
Molecular Polarity Molecular Structure ppt video online download What Bonds Are Non Polar In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent. When the electronegativity difference is. Intermolecular bonds are found between molecules. A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. What is a nonpolar covalent bond? A. What Bonds Are Non Polar.
From pediaa.com
Difference Between Polar and Nonpolar Bonds What Bonds Are Non Polar What is a nonpolar covalent bond? A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. In a nonpolar covalent bond, electrons are equally shared. When the electronegativity difference is. Both of the bonds inside the molecule are polar bonds. A covalent bond that has an unequal sharing of electrons,. What Bonds Are Non Polar.
From www.pinterest.nz
Polar vs. Nonpolar Bonds — Overview & Examples Expii Ionic Bonding What Bonds Are Non Polar A covalent bond that has an equal sharing of. Both of the bonds inside the molecule are polar bonds. In a nonpolar covalent bond, electrons are equally shared. They are also known as van der waals forces, and there are several types to consider. When the electronegativity difference is. A nonpolar covalent bond is a covalent bond in which the. What Bonds Are Non Polar.
From byjus.com
Are non polar N2,H2,O2 covalent bonds strong? What Bonds Are Non Polar A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. Both of the bonds inside the molecule are polar bonds. A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. Intermolecular bonds are found between. What Bonds Are Non Polar.
From www.vrogue.co
Ch4 Polar Or Nonpolar Covalent Bond Ppt The Chemistry vrogue.co What Bonds Are Non Polar A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. In a nonpolar covalent bond, electrons are equally shared. A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. What is a nonpolar covalent bond?. What Bonds Are Non Polar.
From psiberg.com
Polar vs Nonpolar bonds What is the Main Difference? PSIBERG What Bonds Are Non Polar A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. When the electronegativity difference is. A covalent bond that has an equal sharing of. Intermolecular bonds are found between molecules. A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared. What Bonds Are Non Polar.
From www.youtube.com
Polar and Nonpolar Molecules YouTube What Bonds Are Non Polar What is a nonpolar covalent bond? They are also known as van der waals forces, and there are several types to consider. A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. A covalent bond that has an equal sharing of. A covalent bond that has an unequal sharing of. What Bonds Are Non Polar.
From studylib.net
Polar and Nonpolar Molecules What Bonds Are Non Polar What is a nonpolar covalent bond? Both of the bonds inside the molecule are polar bonds. A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. A covalent bond that has an equal sharing of. A covalent bond is formed when two atoms share electrons. What Bonds Are Non Polar.
From www.slideserve.com
PPT Bonding PowerPoint Presentation ID3050946 What Bonds Are Non Polar In a nonpolar covalent bond, electrons are equally shared. A covalent bond that has an equal sharing of. A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a. What Bonds Are Non Polar.
From surfguppy.com
What is Nonpolar Covalent Bond What Bonds Are Non Polar When the electronegativity difference is. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent. Intermolecular bonds are found between molecules. They are also known as van der waals forces, and there are several types to consider. A covalent bond that has an equal sharing of. A nonpolar. What Bonds Are Non Polar.
From www.slideserve.com
PPT Polar and Nonpolar Covalent Bonds PowerPoint Presentation, free What Bonds Are Non Polar In a nonpolar covalent bond, electrons are equally shared. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent. A covalent bond that has an equal sharing of. What is a nonpolar covalent bond? A covalent bond that has an unequal sharing of electrons, as in part (b). What Bonds Are Non Polar.
From stilleducation.com
Polar and Nonpolar Covalent Bonds Characteristics & Differences What Bonds Are Non Polar A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. They are also known as van der waals forces, and there are several types to consider. In a nonpolar covalent bond, electrons are equally shared. Intermolecular bonds are found between molecules. A covalent bond is formed when two atoms share. What Bonds Are Non Polar.
From www.slideserve.com
PPT Polar or Nonpolar PowerPoint Presentation, free download ID3483667 What Bonds Are Non Polar A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent. A covalent bond is formed when two atoms share electrons between them. A. What Bonds Are Non Polar.
From www.slideserve.com
PPT Bonding PowerPoint Presentation ID3050946 What Bonds Are Non Polar A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. When the electronegativity difference is. In a nonpolar covalent bond, electrons are equally shared. What is a nonpolar covalent bond? A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared. What Bonds Are Non Polar.
From simp-link.com
Difference between polar and nonpolar examples What Bonds Are Non Polar A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. Both of the bonds inside the molecule are polar bonds. A covalent bond is formed when two atoms share electrons between them. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is. What Bonds Are Non Polar.
From www.pinterest.co.uk
Polar vs. Nonpolar Bonds — Overview & Examples Expii Covalent What Bonds Are Non Polar A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. What is a nonpolar covalent bond? In a nonpolar covalent bond, electrons are equally shared. When the electronegativity difference is. A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is. What Bonds Are Non Polar.
From sciencenotes.org
Polar and Nonpolar Molecules What Bonds Are Non Polar When the electronegativity difference is. A covalent bond is formed when two atoms share electrons between them. A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent. A. What Bonds Are Non Polar.
From www.pinterest.com
Nonpolar Covalent Bond Easy Science Covalent bonding, Chemistry What Bonds Are Non Polar In a nonpolar covalent bond, electrons are equally shared. They are also known as van der waals forces, and there are several types to consider. A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. Both of the bonds inside the molecule are polar bonds.. What Bonds Are Non Polar.
From www.pinterest.ph
Pin on Science What Bonds Are Non Polar What is a nonpolar covalent bond? They are also known as van der waals forces, and there are several types to consider. Both of the bonds inside the molecule are polar bonds. A covalent bond that has an equal sharing of. A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\),. What Bonds Are Non Polar.
From msjschemclass.blogspot.com
Ms J's Chemistry Class Polar vs NonPolar Covalent Bonds What Bonds Are Non Polar A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. A covalent bond that has an equal sharing of. In a nonpolar covalent bond, electrons are equally shared. When the electronegativity difference is. What is a nonpolar covalent bond? In a diatomic molecule with two. What Bonds Are Non Polar.
From www.chemistrylearner.com
Nonpolar Covalent Bond Definition and Examples What Bonds Are Non Polar A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. When the electronegativity difference is. In a nonpolar covalent bond, electrons are equally shared. A. What Bonds Are Non Polar.
From simp-link.com
Difference between polar and nonpolar examples What Bonds Are Non Polar A covalent bond that has an equal sharing of. In a nonpolar covalent bond, electrons are equally shared. A covalent bond is formed when two atoms share electrons between them. Intermolecular bonds are found between molecules. What is a nonpolar covalent bond? When the electronegativity difference is. They are also known as van der waals forces, and there are several. What Bonds Are Non Polar.
From chem.libretexts.org
9.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts What Bonds Are Non Polar Intermolecular bonds are found between molecules. Both of the bonds inside the molecule are polar bonds. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent. A covalent bond that has an equal sharing of. A nonpolar covalent bond is a covalent bond in which the bonding electrons. What Bonds Are Non Polar.
From sites.google.com
2.2.2 (i,j) Electronegativity and Bond Polarity Ellesmere OCR A level What Bonds Are Non Polar They are also known as van der waals forces, and there are several types to consider. Both of the bonds inside the molecule are polar bonds. When the electronegativity difference is. A covalent bond that has an equal sharing of. A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms.. What Bonds Are Non Polar.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID2516429 What Bonds Are Non Polar In a nonpolar covalent bond, electrons are equally shared. A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. They are also known as van der waals forces, and there are several types to consider. Intermolecular bonds are found between molecules. When the electronegativity difference. What Bonds Are Non Polar.
From general.chemistrysteps.com
Electronegativity and Bond Polarity Chemistry Steps What Bonds Are Non Polar Both of the bonds inside the molecule are polar bonds. A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. A covalent bond is formed when two atoms share electrons between them. What is a nonpolar covalent bond? A covalent bond that has an unequal sharing of electrons, as in. What Bonds Are Non Polar.
From www.slideserve.com
PPT Polar and Nonpolar Covalent Bonds PowerPoint Presentation, free What Bonds Are Non Polar A covalent bond is formed when two atoms share electrons between them. In a nonpolar covalent bond, electrons are equally shared. When the electronegativity difference is. Both of the bonds inside the molecule are polar bonds. What is a nonpolar covalent bond? Intermolecular bonds are found between molecules. In a diatomic molecule with two identical atoms, there is no difference. What Bonds Are Non Polar.
From www.thoughtco.com
What Is a Polar Bond? Definition and Examples What Bonds Are Non Polar They are also known as van der waals forces, and there are several types to consider. A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. In a nonpolar covalent bond, electrons are equally shared. Intermolecular bonds are found between molecules. When the electronegativity difference. What Bonds Are Non Polar.
From nursehub.com
Understanding Types of Chemical Bonds NurseHub What Bonds Are Non Polar A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. In a nonpolar covalent bond, electrons are equally shared. They are also known as van der waals forces, and there are several types to consider. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so. What Bonds Are Non Polar.
From ar.inspiredpencil.com
Polar Covalent Bond Vs Nonpolar Covalent Bond What Bonds Are Non Polar A covalent bond is formed when two atoms share electrons between them. Intermolecular bonds are found between molecules. A covalent bond that has an unequal sharing of electrons, as in part (b) of figure \ (\pageindex {1}\), is called a polar covalent bond. A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between. What Bonds Are Non Polar.
From pediaa.com
Difference Between Polar and Nonpolar Molecules Definition, Formation What Bonds Are Non Polar Intermolecular bonds are found between molecules. Both of the bonds inside the molecule are polar bonds. What is a nonpolar covalent bond? A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. They are also known as van der waals forces, and there are several types to consider. When the. What Bonds Are Non Polar.
From www.chemistrylearner.com
Polar Covalent Bond Definition and Examples What Bonds Are Non Polar When the electronegativity difference is. Intermolecular bonds are found between molecules. Both of the bonds inside the molecule are polar bonds. A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. What is a nonpolar covalent bond? In a nonpolar covalent bond, electrons are equally shared. A covalent bond that. What Bonds Are Non Polar.