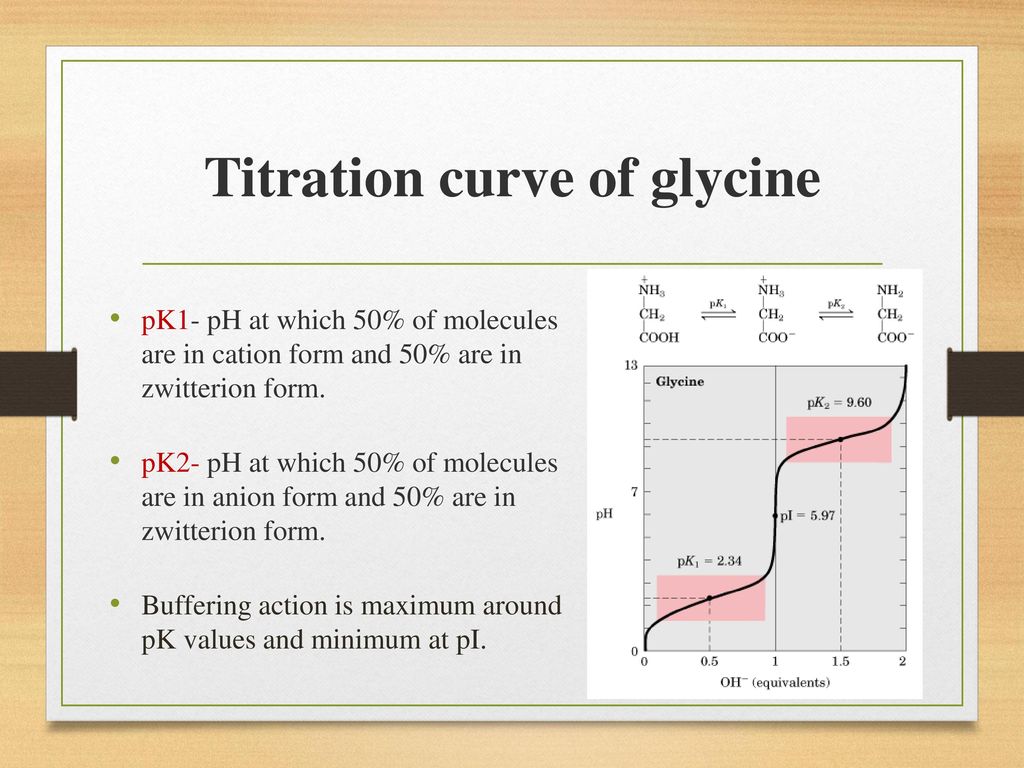
Zwitterion Titration Curve . At a ph lower than 2, both the carboxylate and. If we only add half as much base, only. At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the negatively and. Calculate the concentration of an amino acid. The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and. Write equations for the reaction of the zwitterion of an amino acid with acid and with base. (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. Below is a typical curve for the titration of glycine with naoh.
from slideplayer.com
The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. Write equations for the reaction of the zwitterion of an amino acid with acid and with base. (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. If we only add half as much base, only. Below is a typical curve for the titration of glycine with naoh. At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the negatively and. Calculate the concentration of an amino acid. At a ph lower than 2, both the carboxylate and. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and.
Amino acids (Foundation Block) Dr. Sumbul Fatma. ppt download
Zwitterion Titration Curve At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the negatively and. At a ph lower than 2, both the carboxylate and. (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. Calculate the concentration of an amino acid. Below is a typical curve for the titration of glycine with naoh. At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the negatively and. If we only add half as much base, only. The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. Write equations for the reaction of the zwitterion of an amino acid with acid and with base.
From dokumen.tips
(PDF) to 3(zwitterion) Titration Curve for Alanine Equivalents Zwitterion Titration Curve In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and. Below is a typical curve for the titration of glycine with naoh. Calculate the concentration of an amino acid. Write equations for the reaction of the zwitterion of an amino acid with acid and with base. If. Zwitterion Titration Curve.
From www.numerade.com
SOLVED Draw the titration curve for the dipeptide GLUHISPRO showing Zwitterion Titration Curve Write equations for the reaction of the zwitterion of an amino acid with acid and with base. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding. Zwitterion Titration Curve.
From www.slideserve.com
PPT Amino Acids PowerPoint Presentation, free download ID1842689 Zwitterion Titration Curve In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and. The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. Write equations for the reaction of the zwitterion of an amino acid with acid and with base. Calculate the concentration of an amino acid. At intermediate. Zwitterion Titration Curve.
From aracely-has-zhang.blogspot.com
How to Describe a Titration Curve AracelyhasZhang Zwitterion Titration Curve In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and. At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the negatively and. The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. Below is a typical curve for. Zwitterion Titration Curve.
From slideplayer.com
Biochemistry I L Feb ppt download Zwitterion Titration Curve Below is a typical curve for the titration of glycine with naoh. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and. Calculate the concentration of an amino acid. (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. At intermediate. Zwitterion Titration Curve.
From www.youtube.com
Zwitterion titration Curves YouTube Zwitterion Titration Curve Calculate the concentration of an amino acid. At a ph lower than 2, both the carboxylate and. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. Below is a typical curve for the titration of glycine with naoh. Write. Zwitterion Titration Curve.
From biochemden.com
Titration Curve of Glycine The zwitter ionic changes Zwitterion Titration Curve (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. Below is a typical curve for the titration of glycine with naoh. Write equations. Zwitterion Titration Curve.
From www.numerade.com
SOLVED Refer to the following titration curve of lysine below Which Zwitterion Titration Curve Below is a typical curve for the titration of glycine with naoh. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and. Calculate the concentration of an amino acid. At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the. Zwitterion Titration Curve.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Zwitterion Titration Curve If we only add half as much base, only. At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the negatively and. Write equations for the reaction of the zwitterion of an amino acid with acid and with base. Below is a typical curve for the titration of glycine with naoh. In sum,. Zwitterion Titration Curve.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Zwitterion Titration Curve The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the negatively and. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a. Zwitterion Titration Curve.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Zwitterion Titration Curve (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. Below is a typical curve for the titration of glycine with naoh. At intermediate. Zwitterion Titration Curve.
From warreninstitute.org
Calculating The Isoelectric Point Of Amino Acids And Zwitterions Zwitterion Titration Curve If we only add half as much base, only. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and. Calculate the concentration of an amino acid. At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the negatively and. At. Zwitterion Titration Curve.
From www.numerade.com
SOLVED Zwitterionic form Both groups deprotonated 1 Both groups Zwitterion Titration Curve At a ph lower than 2, both the carboxylate and. (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and. Write equations for the reaction of the zwitterion of an amino. Zwitterion Titration Curve.
From slideplayer.com
Amino acids (Foundation Block) Dr. Sumbul Fatma. ppt download Zwitterion Titration Curve Write equations for the reaction of the zwitterion of an amino acid with acid and with base. (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. Calculate the concentration of an amino acid. At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the negatively and.. Zwitterion Titration Curve.
From www.numerade.com
SOLVED In the titration of the acidic form of an amino acid with NaOH Zwitterion Titration Curve The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. At a ph lower than 2, both the carboxylate and. Below is a typical curve for the titration of glycine with naoh. At intermediate ph's the zwitterion concentration increases, and. Zwitterion Titration Curve.
From www.aqion.de
Zwitterions and Amino Acids Zwitterion Titration Curve Write equations for the reaction of the zwitterion of an amino acid with acid and with base. (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point. Zwitterion Titration Curve.
From www.numerade.com
SOLVED Zwitterionic form Both groups deprotonated 1 Both groups Zwitterion Titration Curve If we only add half as much base, only. Write equations for the reaction of the zwitterion of an amino acid with acid and with base. (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. The shape of a titration curve, a plot of ph versus the amount of acid or base added,. Zwitterion Titration Curve.
From www.researchgate.net
Melting curves for the zwitterionic oligoadenylate 21 with poly (U Zwitterion Titration Curve Write equations for the reaction of the zwitterion of an amino acid with acid and with base. At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the negatively and. (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. If we only add half as much. Zwitterion Titration Curve.
From www.numerade.com
SOLVED In the titration of the acidic form of an amino acid with NaOH Zwitterion Titration Curve If we only add half as much base, only. Below is a typical curve for the titration of glycine with naoh. (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and.. Zwitterion Titration Curve.
From www.youtube.com
Zwitterion/Titration of amino acids/Titration curve of Glycine amino Zwitterion Titration Curve The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. Calculate the concentration of an amino acid. Write equations for the reaction of the zwitterion of an amino acid with acid and with base. At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the negatively and. Below is a typical curve. Zwitterion Titration Curve.
From www.numerade.com
SOLVED In titrating the acidic form of amino acid with NaOH solution Zwitterion Titration Curve Write equations for the reaction of the zwitterion of an amino acid with acid and with base. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and. At a ph lower than 2, both the carboxylate and. At intermediate ph's the zwitterion concentration increases, and at a. Zwitterion Titration Curve.
From mungfali.com
Titration Curve Labeled Zwitterion Titration Curve The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. Write equations for the reaction of the zwitterion of an amino acid with acid and with base. Calculate the. Zwitterion Titration Curve.
From slideplayer.com
Amino Acids (Foundation Block) 1 Lecture Dr. Usman Ghani ppt download Zwitterion Titration Curve Calculate the concentration of an amino acid. At a ph lower than 2, both the carboxylate and. The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. Write equations for the reaction of the zwitterion of an amino acid with acid and with base. The shape of a titration curve, a plot of ph versus the amount of acid. Zwitterion Titration Curve.
From slideplayer.com
Biochemistry I L Feb ppt download Zwitterion Titration Curve The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. If we only add half as much base, only. Below is a typical curve for the titration of glycine with naoh. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and. At intermediate ph's the zwitterion. Zwitterion Titration Curve.
From www.slideserve.com
PPT Amino acids PowerPoint Presentation, free download ID6032806 Zwitterion Titration Curve Below is a typical curve for the titration of glycine with naoh. The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. Calculate the concentration of an amino acid. If we only add half as much base, only. At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the negatively and. (from. Zwitterion Titration Curve.
From www.researchgate.net
Carnosine structure as the cation, zwitterion and anion Download Zwitterion Titration Curve At a ph lower than 2, both the carboxylate and. Write equations for the reaction of the zwitterion of an amino acid with acid and with base. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and. (from elte.prompt.hu) although we often write glycine as nh₂cooh, it. Zwitterion Titration Curve.
From www.numerade.com
SOLVED Zwitterionic form Both groups deprotonated 1 Both groups Zwitterion Titration Curve The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. Below is a typical curve for the titration of glycine with naoh. If we only add half as much base, only. At a ph lower than 2, both the carboxylate and. In sum,. Zwitterion Titration Curve.
From glossary.periodni.com
Zwitterion Chemistry Dictionary & Glossary Zwitterion Titration Curve At a ph lower than 2, both the carboxylate and. If we only add half as much base, only. The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. Calculate the concentration of an amino acid. Write equations for the reaction of the. Zwitterion Titration Curve.
From www.numerade.com
SOLVED 10. The diagram to the right shows an OH titration curve with Zwitterion Titration Curve If we only add half as much base, only. Write equations for the reaction of the zwitterion of an amino acid with acid and with base. At a ph lower than 2, both the carboxylate and. The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. Calculate the concentration of an amino acid. In sum, the titration curve of. Zwitterion Titration Curve.
From oneclass.com
BCHM 218 Lecture Notes Winter 2020, Lecture 1 Titration Curve Zwitterion Titration Curve (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. At a ph lower than 2, both the carboxylate and. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and. If we only add half as much base, only. Calculate the. Zwitterion Titration Curve.
From www.numerade.com
SOLVED The amino acid serine has the following structural formula Zwitterion Titration Curve The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the negatively and. In sum, the titration curve of glycine exhibits two distinct. Zwitterion Titration Curve.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Zwitterion Titration Curve Calculate the concentration of an amino acid. Below is a typical curve for the titration of glycine with naoh. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and. The shape of a titration curve, a plot of ph versus the amount of acid or base added,. Zwitterion Titration Curve.
From www.researchgate.net
Titration curves n(pH), buffer capacity β and its derivative dβ/dpH for Zwitterion Titration Curve At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the negatively and. Calculate the concentration of an amino acid. (from elte.prompt.hu) although we often write glycine as nh₂cooh, it is really a zwitterion, + nh3ch2coo⁻. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of. Zwitterion Titration Curve.
From www.slideserve.com
PPT AMINO ACIDS PowerPoint Presentation ID1205136 Zwitterion Titration Curve Calculate the concentration of an amino acid. Write equations for the reaction of the zwitterion of an amino acid with acid and with base. In sum, the titration curve of glycine exhibits two distinct buffering regions corresponding to the ionisation of its carboxyl and amino groups and. The shape of a titration curve, a plot of ph versus the amount. Zwitterion Titration Curve.
From www.slideserve.com
PPT Date PowerPoint Presentation, free download ID322514 Zwitterion Titration Curve At a ph lower than 2, both the carboxylate and. The titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. If we only add half as much base, only. Calculate the concentration of an amino acid. At intermediate ph's the zwitterion concentration increases, and at a characteristic ph, called the isoelectric point (pi), the negatively and. (from elte.prompt.hu) although. Zwitterion Titration Curve.