Dilution Example Questions . If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. There’s a bottle of 0.750 m nacl on a shelf. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? 10.0 ml of 1.00 m hcl is. You may be given beginning or ending volume and. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution problems, chemistry, molarity & concentration examples, formula & equations The concentration and the volumes change in a dilution. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. This site will produce an unlimited number of practice problems for calulating dilutions.
from www.youtube.com
State whether the concentration of a solution is directly or indirectly proportional to its volume. The concentration and the volumes change in a dilution. You may be given beginning or ending volume and. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. 10.0 ml of 1.00 m hcl is. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution problems, chemistry, molarity & concentration examples, formula & equations There’s a bottle of 0.750 m nacl on a shelf. This site will produce an unlimited number of practice problems for calulating dilutions.
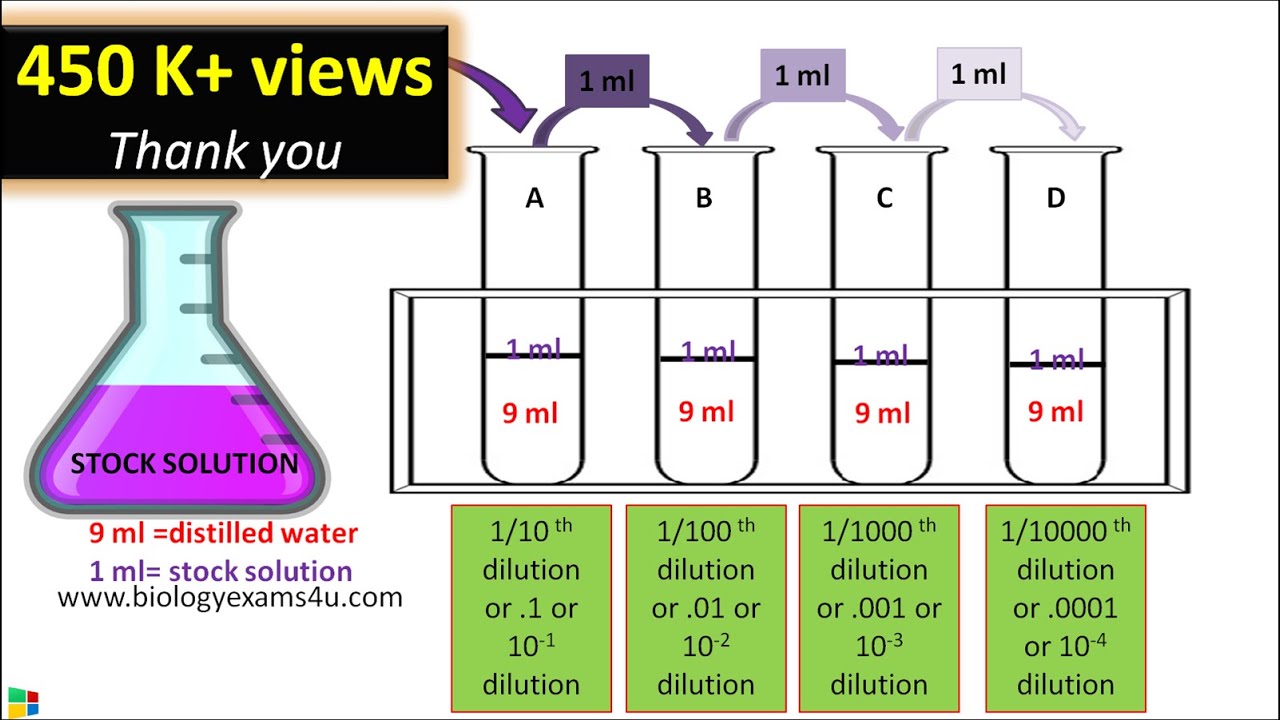
Serial Dilution Method Protocol Step Wise Explanation YouTube
Dilution Example Questions You may be given beginning or ending volume and. 10.0 ml of 1.00 m hcl is. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. You may be given beginning or ending volume and. Dilution problems, chemistry, molarity & concentration examples, formula & equations There’s a bottle of 0.750 m nacl on a shelf. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? The concentration and the volumes change in a dilution. This site will produce an unlimited number of practice problems for calulating dilutions.
From www.chegg.com
Solved Dilution Problems For problems 711 use the dilution Dilution Example Questions You may be given beginning or ending volume and. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Dilution problems, chemistry, molarity & concentration examples, formula & equations The concentration. Dilution Example Questions.
From www.chegg.com
Solved Use the dilution scheme shown below and answer the Dilution Example Questions This site will produce an unlimited number of practice problems for calulating dilutions. The concentration and the volumes change in a dilution. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. You may be given beginning or ending volume and. How much of it do you. Dilution Example Questions.
From www.youtube.com
Quickvideo Using the dilution equation M1V1=M2V2 YouTube Dilution Example Questions Dilution problems, chemistry, molarity & concentration examples, formula & equations The concentration and the volumes change in a dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. This site will produce an unlimited number of practice problems for calulating dilutions. You may be given beginning or ending volume and. How much of it. Dilution Example Questions.
From www.numerade.com
SOLVED Serial Dilution Worksheet Bio152 Paramedical Microbiology Use Dilution Example Questions How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? State whether the concentration of a solution is directly or indirectly proportional to its volume. 10.0 ml of 1.00 m hcl is. This site will produce an unlimited number of practice problems for calulating dilutions. Dilution problems, chemistry, molarity & concentration examples, formula. Dilution Example Questions.
From www.chegg.com
Solved Practice Dilution Problems Section I 1. How much Dilution Example Questions A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. The concentration and the volumes change in a dilution. This site will produce an unlimited. Dilution Example Questions.
From www.chegg.com
Solved Dilution practice problems 1. What is the dilution Dilution Example Questions 10.0 ml of 1.00 m hcl is. This site will produce an unlimited number of practice problems for calulating dilutions. The concentration and the volumes change in a dilution. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? You may be given beginning or ending volume and. Dilution problems, chemistry, molarity &. Dilution Example Questions.
From www.youtube.com
Dilution of Solution Multiple Choice Question Learn how to solve Dilution Example Questions State whether the concentration of a solution is directly or indirectly proportional to its volume. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute.. Dilution Example Questions.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Example Questions If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. This site will produce an unlimited number of practice problems for calulating dilutions. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. You may. Dilution Example Questions.
From www.youtube.com
Dilution Practice Problems & Example Problems YouTube Dilution Example Questions State whether the concentration of a solution is directly or indirectly proportional to its volume. You may be given beginning or ending volume and. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? Dilution problems, chemistry, molarity & concentration examples, formula & equations If you dilute 175 ml of a 1.6 m. Dilution Example Questions.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Example Questions The concentration and the volumes change in a dilution. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. This site will produce an unlimited number of practice problems for calulating dilutions. How much of it do you need to prepare 50 ml of a 0.10 m nacl. Dilution Example Questions.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Example Questions State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution problems, chemistry, molarity & concentration examples, formula & equations There’s a bottle of 0.750 m nacl on a shelf. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? The concentration and the volumes change in a. Dilution Example Questions.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Example Questions You may be given beginning or ending volume and. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. There’s a bottle of 0.750 m nacl on a shelf. 10.0 ml of 1.00 m hcl is. The concentration and the volumes change in a dilution. This site. Dilution Example Questions.
From www.chegg.com
Solved Name Serial Dilution Example Fill out the dilutions Dilution Example Questions The concentration and the volumes change in a dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. There’s a bottle of 0.750 m nacl on a shelf. 10.0 ml of 1.00. Dilution Example Questions.
From www.youtube.com
Video 18 Simple C1V1 = C2V2 dilution calculations YouTube Dilution Example Questions How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. This site will produce an. Dilution Example Questions.
From www.studocu.com
Dilution Practice Dilution Handout SP21 Name Dilution Example Dilution Example Questions State whether the concentration of a solution is directly or indirectly proportional to its volume. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. 10.0 ml of 1.00 m hcl. Dilution Example Questions.
From study.com
Solving Applied Dilution Problems Chemistry Dilution Example Questions 10.0 ml of 1.00 m hcl is. Dilution problems, chemistry, molarity & concentration examples, formula & equations You may be given beginning or ending volume and. The concentration and the volumes change in a dilution. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. How much of. Dilution Example Questions.
From www.chegg.com
Solved In The Image Above, The Final Dilution Is 1 You M... Dilution Example Questions Dilution problems, chemistry, molarity & concentration examples, formula & equations If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilution is a process where the concentration of a solution is lowered. Dilution Example Questions.
From www.numerade.com
SOLVED EXAMPLE 5 DILUTIONS If I wanted to make 100 mL of a 1x buffer Dilution Example Questions How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? You may be given beginning or ending volume and. The concentration and the volumes change in a dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilution is a process where the concentration of a. Dilution Example Questions.
From www.chegg.com
Solved Answer the following questions regarding the dilution Dilution Example Questions You may be given beginning or ending volume and. 10.0 ml of 1.00 m hcl is. The concentration and the volumes change in a dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. Dilution Example Questions.
From www.youtube.com
Serial Dilution Calculations Examples YouTube Dilution Example Questions There’s a bottle of 0.750 m nacl on a shelf. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. This site will produce an unlimited number of practice problems for calulating dilutions. State whether the concentration of a solution is directly or indirectly proportional to its. Dilution Example Questions.
From www.studocu.com
1. Serial Dilution Calculations Dilution Plating Questions Question Dilution Example Questions You may be given beginning or ending volume and. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. This site will produce an unlimited number of practice problems for calulating dilutions.. Dilution Example Questions.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Dilution Example Questions State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. You may be given beginning or ending volume and. How much of it do you need to prepare 50 ml of a. Dilution Example Questions.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Dilution Example Questions Dilution problems, chemistry, molarity & concentration examples, formula & equations How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? This site will produce an unlimited number of practice problems for calulating dilutions. The concentration and the volumes change in a dilution. You may be given beginning or ending volume and. 10.0 ml. Dilution Example Questions.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution Example Questions A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. The concentration and the volumes change in a dilution. Dilution problems, chemistry, molarity & concentration examples, formula & equations You may be given beginning or ending volume and. There’s a bottle of 0.750 m nacl on a. Dilution Example Questions.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Example Questions A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. You may be given beginning or ending volume and. Dilution problems, chemistry, molarity & concentration examples, formula & equations This site will. Dilution Example Questions.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Dilution Example Questions The concentration and the volumes change in a dilution. This site will produce an unlimited number of practice problems for calulating dilutions. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding. Dilution Example Questions.
From www.youtube.com
Dilutions Explained with Problems YouTube Dilution Example Questions This site will produce an unlimited number of practice problems for calulating dilutions. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? State whether the concentration of a solution. Dilution Example Questions.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Example Questions If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. This site will produce an unlimited number of practice problems for calulating dilutions. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. There’s a. Dilution Example Questions.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilution Example Questions State whether the concentration of a solution is directly or indirectly proportional to its volume. This site will produce an unlimited number of practice problems for calulating dilutions. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. There’s a bottle of 0.750 m nacl on a shelf.. Dilution Example Questions.
From www.youtube.com
Solving Dilution Problems in Solution Chemistry CLEAR & SIMPLE YouTube Dilution Example Questions There’s a bottle of 0.750 m nacl on a shelf. You may be given beginning or ending volume and. Dilution problems, chemistry, molarity & concentration examples, formula & equations 10.0 ml of 1.00 m hcl is. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. How. Dilution Example Questions.
From www.slideserve.com
PPT Dilution PowerPoint Presentation, free download ID6016027 Dilution Example Questions A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution problems, chemistry, molarity & concentration examples, formula & equations How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? This site will produce an unlimited number of practice problems. Dilution Example Questions.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Example Questions If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. You may be given beginning or ending volume and. A dilution is a process where the concentration of a solution is lowered by. Dilution Example Questions.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Example Questions This site will produce an unlimited number of practice problems for calulating dilutions. 10.0 ml of 1.00 m hcl is. The concentration and the volumes change in a dilution. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. A dilution is a process where the concentration of. Dilution Example Questions.
From www.youtube.com
How solve dilution and concentration calculation problems YouTube Dilution Example Questions This site will produce an unlimited number of practice problems for calulating dilutions. The concentration and the volumes change in a dilution. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. A dilution is a process where the concentration of a solution is lowered by adding solvent. Dilution Example Questions.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Example Questions A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. This site will produce an unlimited number of practice problems for calulating dilutions. Dilution problems, chemistry, molarity & concentration examples, formula & equations State whether the concentration of a solution is directly or indirectly proportional to its. Dilution Example Questions.