Nitric Oxide Balanced Equation . Balance a chemical equation when given the unbalanced equation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Explain the roles of subscripts and coefficients in chemical equations. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Nitric oxide (radical) + dioxygen = nitrogen dioxide. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. Balanced chemical equation with physical states. It is one of the principal oxides of nitrogen.
from www.numerade.com
Nitric oxide (radical) + dioxygen = nitrogen dioxide. Balance a chemical equation when given the unbalanced equation. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. Balanced chemical equation with physical states. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. It is one of the principal oxides of nitrogen. No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. Explain the roles of subscripts and coefficients in chemical equations.
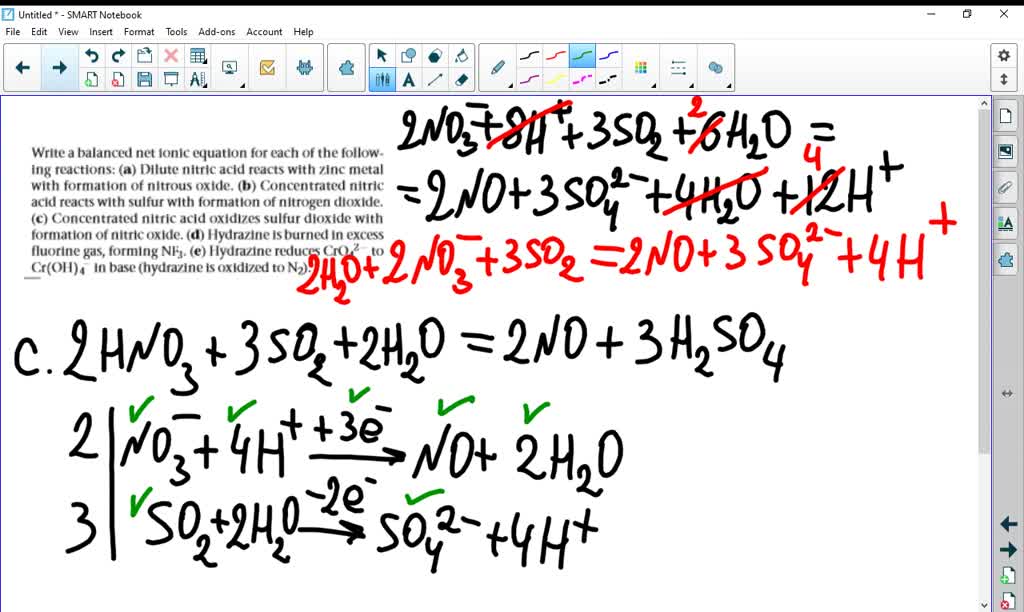
SOLVEDWrite a balanced net ionic equation for each of the following
Nitric Oxide Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the unbalanced equation. It is one of the principal oxides of nitrogen. Nitric oxide (radical) + dioxygen = nitrogen dioxide. No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. Balanced chemical equation with physical states. Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button.
From www.researchgate.net
Chemical equations relevant to the nitric oxide system. Download Nitric Oxide Balanced Equation Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. Balanced chemical equation with physical states. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. It is one of the principal oxides of nitrogen. No + o2 = no2 is a synthesis reaction where two moles of. Nitric Oxide Balanced Equation.
From www.studypool.com
SOLUTION 1) The Nitric oxide shown in the balanced equation is Nitric Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Balanced chemical equation with physical states. Nitric oxide (radical) + dioxygen = nitrogen dioxide. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. Balance a chemical equation when given the unbalanced equation. Write a balanced equation for. Nitric Oxide Balanced Equation.
From oxygengasnaraeru.blogspot.com
Oxygen Gas April 2017 Nitric Oxide Balanced Equation Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. Balance a chemical equation when given the unbalanced equation. No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Nitric oxide. Nitric Oxide Balanced Equation.
From www.youtube.com
Write the balanced chemical equation of the following word equation Nitric Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Balanced chemical equation with physical states. It is one of the principal oxides of nitrogen. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. Nitric oxide (radical) + dioxygen = nitrogen dioxide. Write a balanced equation for. Nitric Oxide Balanced Equation.
From www.vectorstock.com
Nitric oxide molecule skeletal formula Royalty Free Vector Nitric Oxide Balanced Equation Balance a chemical equation when given the unbalanced equation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. It is one of the principal oxides of nitrogen. Nitric oxide (radical) + dioxygen = nitrogen dioxide. Gaseous nitrogen. Nitric Oxide Balanced Equation.
From www.coursehero.com
[Solved] 2) The balanced equation for the reaction between aqueous Nitric Oxide Balanced Equation Nitric oxide (radical) + dioxygen = nitrogen dioxide. Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the unbalanced equation. No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. Balanced chemical equation. Nitric Oxide Balanced Equation.
From www.numerade.com
SOLVEDSilver metal reacts with nitric acid to give silver ion and Nitric Oxide Balanced Equation Balance a chemical equation when given the unbalanced equation. No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Explain. Nitric Oxide Balanced Equation.
From ar.inspiredpencil.com
Nitric Oxide Lewis Structure Nitric Oxide Balanced Equation Nitric oxide (radical) + dioxygen = nitrogen dioxide. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. It is one of the principal oxides of nitrogen. Balanced chemical equation with physical states. Write a balanced equation. Nitric Oxide Balanced Equation.
From brainly.in
Give the balanced equation to prepare Nitric oxide. Which type of oxide Nitric Oxide Balanced Equation Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. Explain the roles of subscripts and coefficients in chemical equations. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Nitric Oxide Balanced Equation.
From hydrogengasgaosube.blogspot.com
Hydrogen Gas Nitric Oxide Gas Reacts With Hydrogen Gas Nitric Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the unbalanced equation. Balanced chemical equation with physical states. It is one of the principal oxides of nitrogen. Nitric oxide (radical) + dioxygen = nitrogen dioxide. Nitric. Nitric Oxide Balanced Equation.
From www.youtube.com
How to Balance HNO3 = H2O + NO2 + O2 (Nitric acid YouTube Nitric Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. Balanced chemical equation with physical states. It is one of the principal oxides of nitrogen. Write a balanced equation for the decomposition of ammonium nitrate to form molecular. Nitric Oxide Balanced Equation.
From www.toppr.com
copper on addition to nitric acid gets converted to gaseous nitric Nitric Oxide Balanced Equation It is one of the principal oxides of nitrogen. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Explain the roles of subscripts and coefficients in chemical equations. To balance a chemical equation, enter an equation. Nitric Oxide Balanced Equation.
From www.numerade.com
SOLVED Write a balanced halfreaction for the oxidation of gaseous Nitric Oxide Balanced Equation No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. Explain the roles of subscripts and coefficients in chemical equations. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Balanced chemical equation with physical states. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which. Nitric Oxide Balanced Equation.
From www.numerade.com
SOLVED Write the net chemical equation for the production of nitric Nitric Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. Balance a chemical equation when given the unbalanced equation. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water.. Nitric Oxide Balanced Equation.
From solvedlib.com
Ammonia reacts with diatomic oxygen to form nitric ox… SolvedLib Nitric Oxide Balanced Equation No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. It is one of the principal oxides of nitrogen. Explain the roles. Nitric Oxide Balanced Equation.
From www.numerade.com
SOLVEDExample Write Out the balanced chemical equation for the Nitric Oxide Balanced Equation Balance a chemical equation when given the unbalanced equation. No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. To balance a chemical equation,. Nitric Oxide Balanced Equation.
From www.numerade.com
SOLVED 1. Write the balanced equations for the following word Nitric Oxide Balanced Equation Balance a chemical equation when given the unbalanced equation. Explain the roles of subscripts and coefficients in chemical equations. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. It is one of the principal oxides of. Nitric Oxide Balanced Equation.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of Nitric Oxide Balanced Equation No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. It is one of the principal oxides of nitrogen. Nitric oxide (radical) + dioxygen = nitrogen dioxide. Balanced chemical equation with physical states. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Balance a chemical equation when. Nitric Oxide Balanced Equation.
From www.numerade.com
SOLVEDSome metals, such as iron, can be oxidized to more than one Nitric Oxide Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Nitric oxide (radical) + dioxygen = nitrogen dioxide. It is one of the principal oxides of nitrogen. Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. To balance a chemical equation, enter an equation of. Nitric Oxide Balanced Equation.
From www.numerade.com
Write balanced chemical equations for each of the following reactions Nitric Oxide Balanced Equation Balanced chemical equation with physical states. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Balance a chemical equation when given the unbalanced equation. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. Explain the roles of subscripts and coefficients in chemical equations. Nitric oxide. Nitric Oxide Balanced Equation.
From www.answersarena.com
[Solved] Balance the equation using the correct coefficie Nitric Oxide Balanced Equation Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation. Nitric Oxide Balanced Equation.
From www.numerade.com
SOLVEDThe mechanism for the reaction of nitrogen dioxide with carbon Nitric Oxide Balanced Equation It is one of the principal oxides of nitrogen. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Balanced chemical equation with physical states. Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. Balance a chemical equation when given the unbalanced equation. Gaseous nitrogen dioxide. Nitric Oxide Balanced Equation.
From www.youtube.com
N2O=N2+O2 Balanced EquationNitrous oxide=Nitrogen+Oxygen Balanced Nitric Oxide Balanced Equation Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. Explain the roles of subscripts and coefficients in chemical equations. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. No +. Nitric Oxide Balanced Equation.
From atonce.com
50 Unbelievable Benefits of Nitric Oxide You Must Know 2024 Nitric Oxide Balanced Equation No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. Explain the roles of subscripts and coefficients in chemical equations. Balanced chemical equation with physical states. Balance a chemical equation when given the unbalanced equation. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. It is. Nitric Oxide Balanced Equation.
From www.numerade.com
SOLVEDSome metals, such as thallium, can be oxidized to more than one Nitric Oxide Balanced Equation Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. Balance a chemical equation when given the unbalanced equation. Balanced chemical equation with physical states. Nitric oxide (radical) + dioxygen = nitrogen dioxide. To balance a chemical equation, enter. Nitric Oxide Balanced Equation.
From www.chegg.com
Solved Suppose the reaction between nitric oxide and oxygen Nitric Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Explain the roles of subscripts and coefficients in chemical equations.. Nitric Oxide Balanced Equation.
From www.chegg.com
Solved Write a balanced equation for the reaction of nitric Nitric Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. Explain the roles of subscripts and coefficients in chemical equations. It is one of the principal oxides of nitrogen. Balance a chemical equation when given the unbalanced. Nitric Oxide Balanced Equation.
From www.researchgate.net
Chemical equations relevant to the nitric oxide system. Download Nitric Oxide Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. It is one of the principal oxides of nitrogen. Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. Balanced chemical equation with physical states. No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. To balance a chemical. Nitric Oxide Balanced Equation.
From www.numerade.com
SOLVED Thank you for your help 1. Write the unbalanced, and balanced Nitric Oxide Balanced Equation No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. It is one of the principal oxides of nitrogen. Balance a chemical equation when given the unbalanced equation. Write a balanced equation for the decomposition of ammonium nitrate to. Nitric Oxide Balanced Equation.
From www.numerade.com
SOLVED Q.G) Write the balanced chemical equations of the following Nitric Oxide Balanced Equation Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. Nitric oxide (radical) + dioxygen = nitrogen dioxide. No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. Explain the roles of subscripts and coefficients in chemical equations. It is one of the principal oxides of nitrogen. To balance a. Nitric Oxide Balanced Equation.
From mungfali.com
Nitric Oxide Lewis Structure Nitric Oxide Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. It is one of the principal oxides of nitrogen. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. Balanced chemical equation with physical states. Nitric oxide. Nitric Oxide Balanced Equation.
From mungfali.com
Balanced Equation For Nitrogen Oxide Nitric Oxide Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Balanced chemical equation with physical states. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Nitric oxide (radical) + dioxygen = nitrogen dioxide. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. No + o2. Nitric Oxide Balanced Equation.
From www.numerade.com
SOLVED The mechanism for the reaction of nitrogen dioxide with carbon Nitric Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble. Nitric oxide (radical) + dioxygen = nitrogen dioxide. Balance a chemical equation when given the unbalanced equation. No + o2 = no2 is a synthesis reaction where two. Nitric Oxide Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced net ionic equation for each of the following Nitric Oxide Balanced Equation Balance a chemical equation when given the unbalanced equation. No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. Balanced chemical equation with physical states. Nitric oxide (nitrogen oxide or nitrogen monoxide [1]) is a colorless gas with the formula no. Nitric oxide (radical) + dioxygen = nitrogen dioxide. Explain the roles of subscripts and. Nitric Oxide Balanced Equation.
From www.numerade.com
SOLVEDA proposed mechanism for the oxidation of nitric oxide to Nitric Oxide Balanced Equation No + o2 = no2 is a synthesis reaction where two moles of nitric oxide. It is one of the principal oxides of nitrogen. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Balance. Nitric Oxide Balanced Equation.