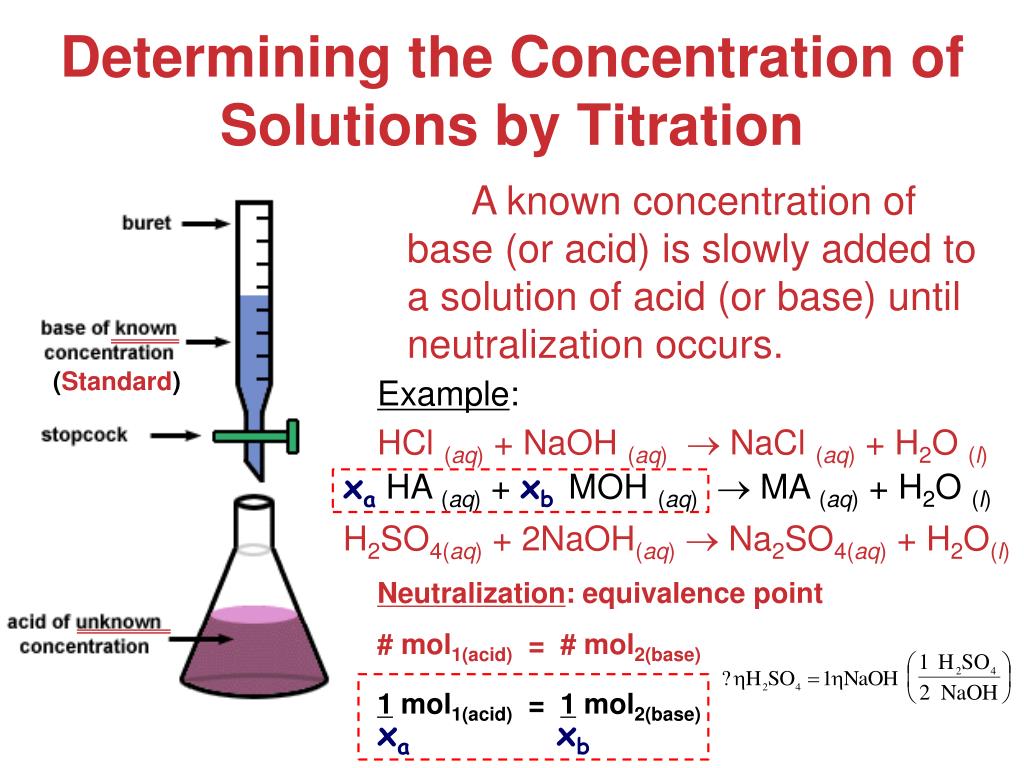
How To Calculate The Concentration Of Naoh In A Titration . M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: suppose that a titration is performed and \(20.70 \: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. The former quantity could be obtained. to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. \ce{naoh}\) is required to reach the end.
from www.slideserve.com
In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. The former quantity could be obtained.
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation
How To Calculate The Concentration Of Naoh In A Titration M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. The former quantity could be obtained. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base:
From www.chegg.com
Solved Calculate the NaOH concentration necessary to How To Calculate The Concentration Of Naoh In A Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. The former quantity could be obtained. suppose that a titration is performed and \(20.70 \: calculate the ph for the strong. How To Calculate The Concentration Of Naoh In A Titration.
From dieggclteco.blob.core.windows.net
Titration Molarity Equation at Marion Moore blog How To Calculate The Concentration Of Naoh In A Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. The former quantity could be obtained. to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with. How To Calculate The Concentration Of Naoh In A Titration.
From www.numerade.com
Titration for Acetic Acid in Vinegar Lab Report Exercise 1 How To Calculate The Concentration Of Naoh In A Titration calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. M_av_a = m_bv_b let's assume you are. How To Calculate The Concentration Of Naoh In A Titration.
From www.numerade.com
SOLVEDCalculate the concentration (in molarity) of an mathrm{NaOH How To Calculate The Concentration Of Naoh In A Titration The former quantity could be obtained. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: \ce{naoh}\) is required to reach the end. . How To Calculate The Concentration Of Naoh In A Titration.
From freeonlinehelpns.blogspot.com
Free Online Help QThe titration of 10.00 mL of a diprotic acid How To Calculate The Concentration Of Naoh In A Titration to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and. How To Calculate The Concentration Of Naoh In A Titration.
From oneclass.com
OneClass Moles of NaOH used in titration mole NaOH Moles of HC2H3O2 How To Calculate The Concentration Of Naoh In A Titration suppose that a titration is performed and \(20.70 \: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: \ce{naoh}\) is required to reach the end. The former quantity could be obtained. In a titration, 25.0 cm 3. How To Calculate The Concentration Of Naoh In A Titration.
From fity.club
Titration Of Hcl With Naoh How To Calculate The Concentration Of Naoh In A Titration M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: \ce{naoh}\) is required to reach the end. to calculate concentration,. How To Calculate The Concentration Of Naoh In A Titration.
From www.tutordale.com
How To Calculate Concentration In Chemistry How To Calculate The Concentration Of Naoh In A Titration to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. The former quantity could be obtained. \ce{naoh}\) is required to reach the end. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. M_av_a = m_bv_b let's assume you are titrating a strong. How To Calculate The Concentration Of Naoh In A Titration.
From fity.club
Titration Calculations How To Calculate The Concentration Of Naoh In A Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to. How To Calculate The Concentration Of Naoh In A Titration.
From exocnnftw.blob.core.windows.net
How Do You Calculate The Concentration Of Naoh In A Titration at How To Calculate The Concentration Of Naoh In A Titration suppose that a titration is performed and \(20.70 \: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. calculate the ph for the strong acid/strong base titration between 50.0 ml. How To Calculate The Concentration Of Naoh In A Titration.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation How To Calculate The Concentration Of Naoh In A Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. The former quantity could be obtained. suppose that a titration is performed and \(20.70 \: M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. \ce{naoh}\) is required to reach the end. to calculate concentration,. How To Calculate The Concentration Of Naoh In A Titration.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration How To Calculate The Concentration Of Naoh In A Titration to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: In a titration, 25.0 cm 3 of. How To Calculate The Concentration Of Naoh In A Titration.
From www.coursehero.com
[Solved] Determine the concentration of the NaOH titrant by using your How To Calculate The Concentration Of Naoh In A Titration M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. The former quantity could be. How To Calculate The Concentration Of Naoh In A Titration.
From www.youtube.com
find out concentration of naoh in water by titration method NaOH in How To Calculate The Concentration Of Naoh In A Titration calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. M_av_a = m_bv_b let's assume you are. How To Calculate The Concentration Of Naoh In A Titration.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas How To Calculate The Concentration Of Naoh In A Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. suppose that a titration is performed and \(20.70 \: The former quantity could be obtained. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of. How To Calculate The Concentration Of Naoh In A Titration.
From fr.scribd.com
Calculating The Concentration of NaOH Solution Using Titration How To Calculate The Concentration Of Naoh In A Titration calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. to calculate concentration, we need to know the amount of naoh and the. How To Calculate The Concentration Of Naoh In A Titration.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube How To Calculate The Concentration Of Naoh In A Titration to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. \ce{naoh}\) is required to reach. How To Calculate The Concentration Of Naoh In A Titration.
From learningschoolmysticis9.z22.web.core.windows.net
How To Do Titrations In Chemistry How To Calculate The Concentration Of Naoh In A Titration M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. \ce{naoh}\) is required to reach the end. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. suppose that a titration is performed and \(20.70 \: to calculate concentration, we need to know the amount. How To Calculate The Concentration Of Naoh In A Titration.
From exocnnftw.blob.core.windows.net
How Do You Calculate The Concentration Of Naoh In A Titration at How To Calculate The Concentration Of Naoh In A Titration suppose that a titration is performed and \(20.70 \: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. \ce{naoh}\). How To Calculate The Concentration Of Naoh In A Titration.
From exocnnftw.blob.core.windows.net
How Do You Calculate The Concentration Of Naoh In A Titration at How To Calculate The Concentration Of Naoh In A Titration calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: suppose that a titration is performed and \(20.70 \: to calculate concentration, we need to know the amount of naoh and the volume of solution in which. How To Calculate The Concentration Of Naoh In A Titration.
From quizspattering.z21.web.core.windows.net
How To Find Equivalence Point Of Titration How To Calculate The Concentration Of Naoh In A Titration to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. The former quantity could be obtained. suppose that a titration is performed and \(20.70 \: In a titration, 25.0. How To Calculate The Concentration Of Naoh In A Titration.
From exommzgkk.blob.core.windows.net
Titration Concentration Of Solution at Tony Pelletier blog How To Calculate The Concentration Of Naoh In A Titration \ce{naoh}\) is required to reach the end. to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. The former quantity could be obtained. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. M_av_a = m_bv_b let's assume you are titrating a strong. How To Calculate The Concentration Of Naoh In A Titration.
From www.numerade.com
SOLVED The following data were collected during a titration Calculate How To Calculate The Concentration Of Naoh In A Titration suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. In a titration,. How To Calculate The Concentration Of Naoh In A Titration.
From fity.club
Titration Calculations How To Calculate The Concentration Of Naoh In A Titration to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. suppose that a titration is performed and \(20.70 \: The former quantity could be obtained. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m. How To Calculate The Concentration Of Naoh In A Titration.
From www.numerade.com
SOLVED Part III Titration Standardization of NaOH solution (finding How To Calculate The Concentration Of Naoh In A Titration to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: In a titration, 25.0 cm 3 of. How To Calculate The Concentration Of Naoh In A Titration.
From learningschoolregibanb.z21.web.core.windows.net
Chemistry Titration Questions And Answers How To Calculate The Concentration Of Naoh In A Titration to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. suppose that a titration is performed and \(20.70 \: The former quantity could be obtained. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m. How To Calculate The Concentration Of Naoh In A Titration.
From www.chegg.com
Solved What is the average concentration of your How To Calculate The Concentration Of Naoh In A Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. M_av_a = m_bv_b let's assume you. How To Calculate The Concentration Of Naoh In A Titration.
From www.vrogue.co
Titration Practical And Calculation Naoh And Hcl Yout vrogue.co How To Calculate The Concentration Of Naoh In A Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. The former quantity could be obtained. suppose that a titration is performed and \(20.70 \: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of. How To Calculate The Concentration Of Naoh In A Titration.
From fity.club
Concentration Formula How To Calculate The Concentration Of Naoh In A Titration calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. The former quantity could be obtained. \ce{naoh}\). How To Calculate The Concentration Of Naoh In A Titration.
From fity.club
Titration Of Hcl With Naoh How To Calculate The Concentration Of Naoh In A Titration The former quantity could be obtained. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: suppose that a titration is performed and \(20.70 \: to calculate concentration, we need to know the amount of naoh and. How To Calculate The Concentration Of Naoh In A Titration.
From www.youtube.com
The titration of 25 0 mL of an unknown concentration of H2SO4 solution How To Calculate The Concentration Of Naoh In A Titration to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. \ce{naoh}\) is required to reach the end. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base:. How To Calculate The Concentration Of Naoh In A Titration.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation How To Calculate The Concentration Of Naoh In A Titration \ce{naoh}\) is required to reach the end. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: suppose that a titration is performed. How To Calculate The Concentration Of Naoh In A Titration.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry How To Calculate The Concentration Of Naoh In A Titration \ce{naoh}\) is required to reach the end. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and. How To Calculate The Concentration Of Naoh In A Titration.
From fity.club
Titration Of Hcl With Naoh How To Calculate The Concentration Of Naoh In A Titration calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium. How To Calculate The Concentration Of Naoh In A Titration.
From www.researchgate.net
NaOH concentration, titration volume and pH value Download Table How To Calculate The Concentration Of Naoh In A Titration The former quantity could be obtained. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. How To Calculate The Concentration Of Naoh In A Titration.