Titration Problems Chegg . A 0.0100 l sample of h_2so_4 was titrated with 0.150 m naoh. Complete the titration problems below. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. Titration #1 a total of 25.0 ml of 0.150 m potassium hydroxide (koh) was required. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. All ten of the above examples are multi. What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x 10¯ 10) with 0.100 m naoh?.
from www.chegg.com
the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. A 0.0100 l sample of h_2so_4 was titrated with 0.150 m naoh. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. All ten of the above examples are multi. example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. Complete the titration problems below. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x 10¯ 10) with 0.100 m naoh?. Titration #1 a total of 25.0 ml of 0.150 m potassium hydroxide (koh) was required.
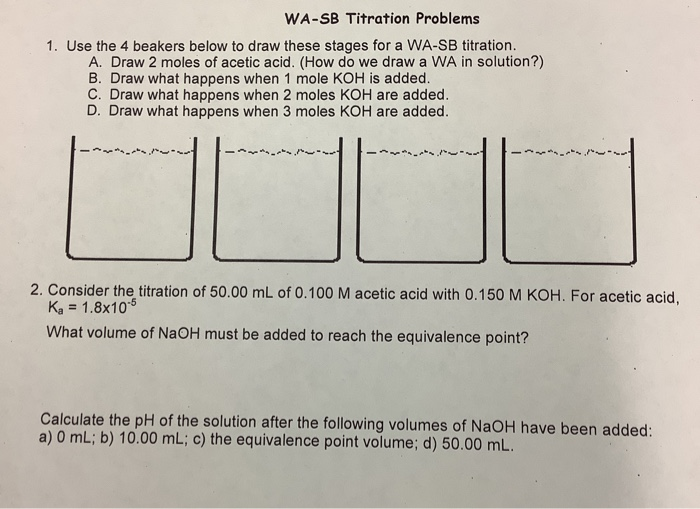
Solved WASB Titration Problems 1. Use the 4 beakers below
Titration Problems Chegg the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. All ten of the above examples are multi. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. A 0.0100 l sample of h_2so_4 was titrated with 0.150 m naoh. example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. Titration #1 a total of 25.0 ml of 0.150 m potassium hydroxide (koh) was required. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x 10¯ 10) with 0.100 m naoh?. Complete the titration problems below.
From www.chegg.com
Solved AcidBase Titration Please help! Explain each step Titration Problems Chegg in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. All ten of the above examples are multi. example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. Titration. Titration Problems Chegg.
From www.chegg.com
Solved Here is a titration curve for 10.00 mL of an unknown Titration Problems Chegg Titration #1 a total of 25.0 ml of 0.150 m potassium hydroxide (koh) was required. A 0.0100 l sample of h_2so_4 was titrated with 0.150 m naoh. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. Complete the titration problems below. the example below demonstrates the technique to. Titration Problems Chegg.
From www.chegg.com
Solved Name Date, Titration Lab Sheet Day 2 (Alternate) Titration Problems Chegg All ten of the above examples are multi. Titration #1 a total of 25.0 ml of 0.150 m potassium hydroxide (koh) was required. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid. Titration Problems Chegg.
From www.chegg.com
Solved Acidbase Titration calculations (Show work) Titration Problems Chegg in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. A 0.0100 l sample of h_2so_4 was titrated with 0.150 m naoh. What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k. Titration Problems Chegg.
From www.chegg.com
Solved Problem Set 9 Titrations and pH Name Date Lab Titration Problems Chegg What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x 10¯ 10) with 0.100 m naoh?. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. Complete the titration problems below. Titration #1 a total of 25.0 ml. Titration Problems Chegg.
From www.chegg.com
Solved The titration curve shown below represents a 25 mL Titration Problems Chegg the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. Titration #1 a total of 25.0 ml of 0.150 m potassium hydroxide (koh) was required. All ten of the above examples are multi. in this section, we will see how to perform calculations to predict the ph at any point. Titration Problems Chegg.
From www.chegg.com
Solved REPORT SHEET Experiment 11 AcidBase Titration Titration Problems Chegg Titration #1 a total of 25.0 ml of 0.150 m potassium hydroxide (koh) was required. All ten of the above examples are multi. example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. A 0.0100 l sample of h_2so_4 was titrated with 0.150 m naoh. the example. Titration Problems Chegg.
From www.chegg.com
Solved Use Table 1 titration data for standardization of Titration Problems Chegg example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x 10¯ 10) with 0.100 m naoh?. Complete the titration problems below. Titration #1 a total. Titration Problems Chegg.
From www.chegg.com
Solved Fit Page Height 8* Sources of Error in Titrations Titration Problems Chegg the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Complete the titration problems below. Titration #1 a total of 25.0 ml. Titration Problems Chegg.
From www.chegg.com
Solved AcidBase Titration Practice Problems 1) Calculate Titration Problems Chegg Complete the titration problems below. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. All ten of the above examples are. Titration Problems Chegg.
From www.chegg.com
Solved WASB Titration Problems 1. Use the 4 beakers below Titration Problems Chegg Complete the titration problems below. example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x 10¯ 10) with 0.100 m naoh?. A 0.0100 l sample. Titration Problems Chegg.
From www.chegg.com
Solved Titrations Practice WorksheetFxtra practice Find the Titration Problems Chegg Complete the titration problems below. example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x 10¯ 10) with 0.100 m naoh?. All ten of the. Titration Problems Chegg.
From www.chegg.com
I need help with a titration lab. I how to Titration Problems Chegg example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. the example below demonstrates the technique to solve. Titration Problems Chegg.
From www.chegg.com
Solved Problem IV Titrations[IV1] It takes 33.5mL of Titration Problems Chegg the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. Titration #1 a total of 25.0 ml of 0.150 m potassium hydroxide (koh) was required. What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x 10¯ 10). Titration Problems Chegg.
From www.chegg.com
Solved KHP Titration Report Sheet Concentration of NaOH Titration Problems Chegg example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. All ten of the above examples are multi. Complete the titration problems below. What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x 10¯ 10). Titration Problems Chegg.
From www.chegg.com
Solved A student performed three aqueous layer titrations in Titration Problems Chegg the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. All ten of the above examples are multi. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. example 10 is the titration of the salt of a weak acid. Titration Problems Chegg.
From www.chegg.com
Solved Exercise 16.71 Problems by Topic Titrations, pH Titration Problems Chegg All ten of the above examples are multi. example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. A 0.0100 l sample of h_2so_4 was titrated with 0.150 m naoh. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid. Titration Problems Chegg.
From www.chegg.com
Solved REPORT.SHEETS Experiment 9 Titration of Vinegar Titration Problems Chegg in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. What is the ph at the equivalence point in the titration. Titration Problems Chegg.
From cewuuttv.blob.core.windows.net
Titration Math Problems at Joy Rodriguez blog Titration Problems Chegg A 0.0100 l sample of h_2so_4 was titrated with 0.150 m naoh. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x 10¯ 10) with 0.100 m. Titration Problems Chegg.
From www.chegg.com
Solved This is a part of the 6 question titration problem. Titration Problems Chegg A 0.0100 l sample of h_2so_4 was titrated with 0.150 m naoh. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. All ten of the above examples are multi. example 10 is the titration of the salt of a. Titration Problems Chegg.
From www.chegg.com
Solved REPORT SHEET AcidBase Titration LAB 10 м A. Titration Problems Chegg Titration #1 a total of 25.0 ml of 0.150 m potassium hydroxide (koh) was required. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. in this section, we will. Titration Problems Chegg.
From www.chegg.com
Solved 1. Answer the following questions using the titration Titration Problems Chegg Complete the titration problems below. Titration #1 a total of 25.0 ml of 0.150 m potassium hydroxide (koh) was required. example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid. Titration Problems Chegg.
From www.chegg.com
Solved Titration Problems Prepare 50.00 mL portions of Titration Problems Chegg Complete the titration problems below. A 0.0100 l sample of h_2so_4 was titrated with 0.150 m naoh. Titration #1 a total of 25.0 ml of 0.150 m potassium hydroxide (koh) was required. All ten of the above examples are multi. example 10 is the titration of the salt of a weak acid (making the salt a base) with a. Titration Problems Chegg.
From www.chegg.com
Solved Titration Curves Worksheet The following are Weak Titration Problems Chegg What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x 10¯ 10) with 0.100 m naoh?. All ten of the above examples are multi. Complete the titration problems below. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid. Titration Problems Chegg.
From www.chegg.com
Solved Practice Problems for AcidBase titration. Find the Titration Problems Chegg the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. A 0.0100 l sample of h_2so_4 was titrated with 0.150 m naoh. Complete the titration problems below. What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x. Titration Problems Chegg.
From www.chegg.com
Solved To review how titration calculations work, here's a Titration Problems Chegg Complete the titration problems below. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. A 0.0100 l sample of h_2so_4 was titrated with 0.150 m naoh. All ten. Titration Problems Chegg.
From www.chegg.com
Solved Given the following titration curve answer questions Titration Problems Chegg Titration #1 a total of 25.0 ml of 0.150 m potassium hydroxide (koh) was required. example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. What is the ph. Titration Problems Chegg.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Titration Problems Chegg Titration #1 a total of 25.0 ml of 0.150 m potassium hydroxide (koh) was required. Complete the titration problems below. What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x 10¯ 10) with 0.100 m naoh?. example 10 is the titration of the salt of a. Titration Problems Chegg.
From www.chegg.com
Solved Please help titration problem. Titration Problems Chegg the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. Complete the titration problems below. example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. in this section, we will see how to perform calculations to predict the. Titration Problems Chegg.
From www.chegg.com
Solved unit 11 8. Work the following titration problems. Titration Problems Chegg What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x 10¯ 10) with 0.100 m naoh?. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. A 0.0100. Titration Problems Chegg.
From www.chegg.com
Solved Titrations Workahoot For each problem, do the Titration Problems Chegg in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. A 0.0100 l sample of h_2so_4 was titrated with 0.150 m naoh.. Titration Problems Chegg.
From www.chegg.com
Solved A "titration" problem. Calculate the pH of a solution Titration Problems Chegg A 0.0100 l sample of h_2so_4 was titrated with 0.150 m naoh. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the.. Titration Problems Chegg.
From www.chegg.com
Solved Colorimetric Titration Experiment Determine the Titration Problems Chegg in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Titration #1 a total of 25.0 ml of 0.150 m potassium hydroxide (koh) was required. All ten of the above examples are multi. What is the ph at the equivalence point. Titration Problems Chegg.
From www.chegg.com
Solved Consider the titration curve given below. This Titration Problems Chegg the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x 10¯ 10) with 0.100 m naoh?. All ten of the above examples are multi. in this. Titration Problems Chegg.
From www.chegg.com
Solved Part 2 Consider the titration curve in Problem 1 and Titration Problems Chegg Complete the titration problems below. What is the ph at the equivalence point in the titration of 100.0 ml of 0.100 m hcn (k a = 4.9 x 10¯ 10) with 0.100 m naoh?. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. in this section, we will. Titration Problems Chegg.