Why Is Iron Reactive . In a very finely divided state metallic iron is pyrophoric (i.e., it ignites spontaneously). The element iron is a very chemically reactive metal, and reacts openly with oxygen in humid air; Learn why iron is not very reactive, but compared to other common metals, it is. It combines vigorously with chlorine on mild heating and. Once the sacrificial metal has corroded away, it can simply be replaced. Pure iron is quite reactive. See the full reactivity series, the atomic structure of iron, and the formation and oxidation of iron. Learn about the properties, reactions and uses of iron and its compounds. Find out how iron (iii) hydroxide is formed and. The more reactive metal oxidises more readily than iron, so it ‘sacrifices’ itself while the iron does not rust. Iron dissolves in water, and reacts. The lower atomic number gives the iron a higher electron affinity than zinc, which makes it more reactive than its counterpart. Iron has more potential to form bonds with. 58 rows learn about the reactivity series of metals, an empirical and analytical progression of their reactivity with water, acids and other. In fact, free iron is not readily found in nature.
from www.teachoo.com
Iron has more potential to form bonds with. The lower atomic number gives the iron a higher electron affinity than zinc, which makes it more reactive than its counterpart. Iron dissolves in water, and reacts. See the full reactivity series, the atomic structure of iron, and the formation and oxidation of iron. Find out how iron (iii) hydroxide is formed and. Pure iron is quite reactive. Once the sacrificial metal has corroded away, it can simply be replaced. In a very finely divided state metallic iron is pyrophoric (i.e., it ignites spontaneously). 58 rows learn about the reactivity series of metals, an empirical and analytical progression of their reactivity with water, acids and other. The element iron is a very chemically reactive metal, and reacts openly with oxygen in humid air;
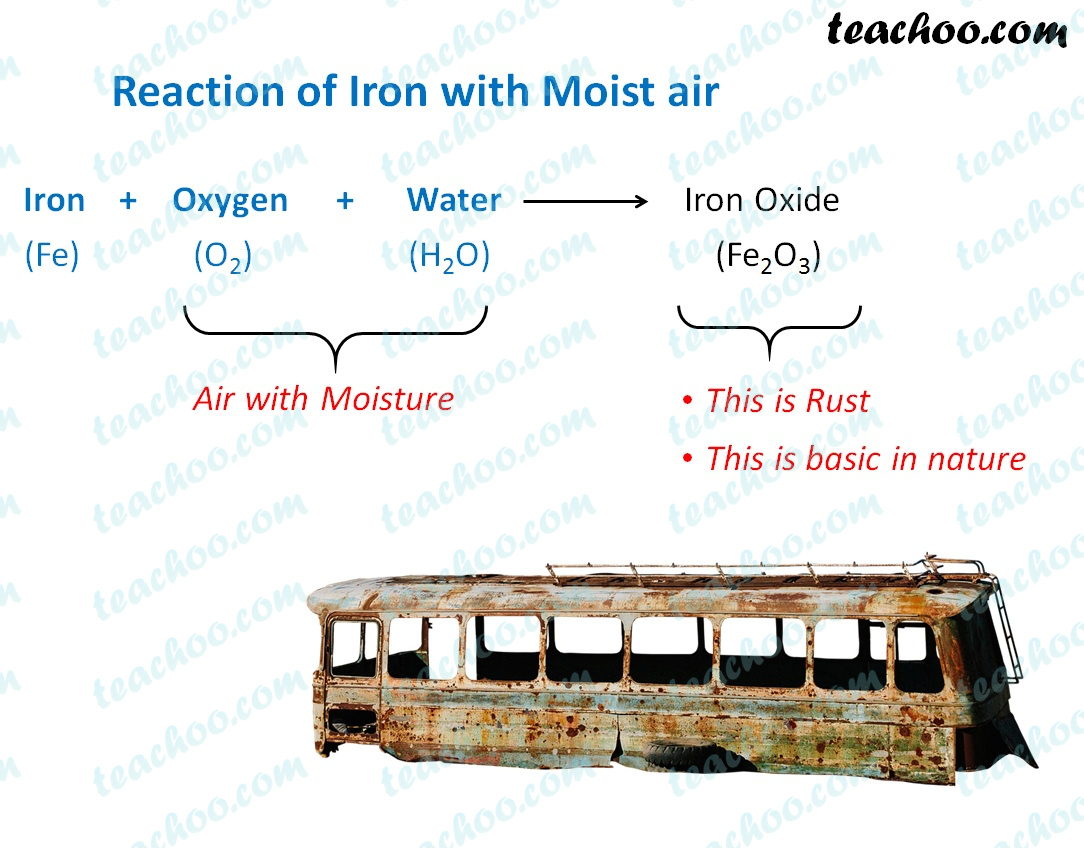
Reaction of Metals and Nonmetals with Oxygen Concepts
Why Is Iron Reactive See the full reactivity series, the atomic structure of iron, and the formation and oxidation of iron. In fact, free iron is not readily found in nature. Learn about the properties, reactions and uses of iron and its compounds. 58 rows learn about the reactivity series of metals, an empirical and analytical progression of their reactivity with water, acids and other. See the full reactivity series, the atomic structure of iron, and the formation and oxidation of iron. The lower atomic number gives the iron a higher electron affinity than zinc, which makes it more reactive than its counterpart. In a very finely divided state metallic iron is pyrophoric (i.e., it ignites spontaneously). Iron dissolves in water, and reacts. Pure iron is quite reactive. The element iron is a very chemically reactive metal, and reacts openly with oxygen in humid air; Find out how iron (iii) hydroxide is formed and. The more reactive metal oxidises more readily than iron, so it ‘sacrifices’ itself while the iron does not rust. It combines vigorously with chlorine on mild heating and. Learn why iron is not very reactive, but compared to other common metals, it is. Iron has more potential to form bonds with. Once the sacrificial metal has corroded away, it can simply be replaced.
From melscience.com
Characteristics of iron and its reaction with oxygen MEL Chemistry Why Is Iron Reactive In a very finely divided state metallic iron is pyrophoric (i.e., it ignites spontaneously). Learn why iron is not very reactive, but compared to other common metals, it is. See the full reactivity series, the atomic structure of iron, and the formation and oxidation of iron. In fact, free iron is not readily found in nature. The element iron is. Why Is Iron Reactive.
From askhematologist.com
Prepare for MRCP Examination Ask Hematologist Understand Hematology Why Is Iron Reactive Pure iron is quite reactive. It combines vigorously with chlorine on mild heating and. Once the sacrificial metal has corroded away, it can simply be replaced. Learn why iron is not very reactive, but compared to other common metals, it is. Learn about the properties, reactions and uses of iron and its compounds. In a very finely divided state metallic. Why Is Iron Reactive.
From chem.libretexts.org
20.8 Corrosion Chemistry LibreTexts Why Is Iron Reactive In a very finely divided state metallic iron is pyrophoric (i.e., it ignites spontaneously). Find out how iron (iii) hydroxide is formed and. The element iron is a very chemically reactive metal, and reacts openly with oxygen in humid air; The more reactive metal oxidises more readily than iron, so it ‘sacrifices’ itself while the iron does not rust. Iron. Why Is Iron Reactive.
From www.teachoo.com
The Chemical Reaction Involved in the Corrosion of Iron Metal Why Is Iron Reactive Find out how iron (iii) hydroxide is formed and. In fact, free iron is not readily found in nature. Learn why iron is not very reactive, but compared to other common metals, it is. The element iron is a very chemically reactive metal, and reacts openly with oxygen in humid air; The lower atomic number gives the iron a higher. Why Is Iron Reactive.
From www.youtube.com
Reactions of Iron Reactions Chemistry FuseSchool YouTube Why Is Iron Reactive See the full reactivity series, the atomic structure of iron, and the formation and oxidation of iron. In fact, free iron is not readily found in nature. Learn why iron is not very reactive, but compared to other common metals, it is. 58 rows learn about the reactivity series of metals, an empirical and analytical progression of their reactivity with. Why Is Iron Reactive.
From www.misslittledoc.com
Why Iron is Important ⋆ Why Is Iron Reactive Pure iron is quite reactive. The element iron is a very chemically reactive metal, and reacts openly with oxygen in humid air; Learn why iron is not very reactive, but compared to other common metals, it is. In fact, free iron is not readily found in nature. Find out how iron (iii) hydroxide is formed and. Iron dissolves in water,. Why Is Iron Reactive.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID5823759 Why Is Iron Reactive Pure iron is quite reactive. In a very finely divided state metallic iron is pyrophoric (i.e., it ignites spontaneously). Iron dissolves in water, and reacts. Once the sacrificial metal has corroded away, it can simply be replaced. The more reactive metal oxidises more readily than iron, so it ‘sacrifices’ itself while the iron does not rust. 58 rows learn about. Why Is Iron Reactive.
From ar.inspiredpencil.com
Rusting Of Iron Diagram Why Is Iron Reactive In fact, free iron is not readily found in nature. Find out how iron (iii) hydroxide is formed and. Iron has more potential to form bonds with. See the full reactivity series, the atomic structure of iron, and the formation and oxidation of iron. The lower atomic number gives the iron a higher electron affinity than zinc, which makes it. Why Is Iron Reactive.
From sciencenotes.org
Thermite Reaction Chemistry Demonstration Why Is Iron Reactive The more reactive metal oxidises more readily than iron, so it ‘sacrifices’ itself while the iron does not rust. Iron dissolves in water, and reacts. Once the sacrificial metal has corroded away, it can simply be replaced. It combines vigorously with chlorine on mild heating and. Pure iron is quite reactive. Learn why iron is not very reactive, but compared. Why Is Iron Reactive.
From en.ppt-online.org
Metals online presentation Why Is Iron Reactive 58 rows learn about the reactivity series of metals, an empirical and analytical progression of their reactivity with water, acids and other. Once the sacrificial metal has corroded away, it can simply be replaced. The more reactive metal oxidises more readily than iron, so it ‘sacrifices’ itself while the iron does not rust. Iron has more potential to form bonds. Why Is Iron Reactive.
From www.slideserve.com
PPT Magnesium reacts quicker than copper and iron. There is a league Why Is Iron Reactive Learn about the properties, reactions and uses of iron and its compounds. In fact, free iron is not readily found in nature. Once the sacrificial metal has corroded away, it can simply be replaced. It combines vigorously with chlorine on mild heating and. The more reactive metal oxidises more readily than iron, so it ‘sacrifices’ itself while the iron does. Why Is Iron Reactive.
From getrevising.co.uk
The Reactivity Series Revision Notes in GCSE Chemistry Why Is Iron Reactive It combines vigorously with chlorine on mild heating and. Learn why iron is not very reactive, but compared to other common metals, it is. The lower atomic number gives the iron a higher electron affinity than zinc, which makes it more reactive than its counterpart. Learn about the properties, reactions and uses of iron and its compounds. Find out how. Why Is Iron Reactive.
From mrcartlidgesaigon.edublogs.org
C10 Metals Science) Mr Cartlidge's second Science Blog Why Is Iron Reactive Iron has more potential to form bonds with. It combines vigorously with chlorine on mild heating and. The element iron is a very chemically reactive metal, and reacts openly with oxygen in humid air; Iron dissolves in water, and reacts. See the full reactivity series, the atomic structure of iron, and the formation and oxidation of iron. 58 rows learn. Why Is Iron Reactive.
From smstews.weebly.com
Iron reactivity smstews Why Is Iron Reactive In a very finely divided state metallic iron is pyrophoric (i.e., it ignites spontaneously). The element iron is a very chemically reactive metal, and reacts openly with oxygen in humid air; Find out how iron (iii) hydroxide is formed and. Pure iron is quite reactive. Iron has more potential to form bonds with. Iron dissolves in water, and reacts. See. Why Is Iron Reactive.
From selftution.com
Reactivity Series of Metals and Nonmetals » Selftution Why Is Iron Reactive 58 rows learn about the reactivity series of metals, an empirical and analytical progression of their reactivity with water, acids and other. Find out how iron (iii) hydroxide is formed and. It combines vigorously with chlorine on mild heating and. Learn why iron is not very reactive, but compared to other common metals, it is. Learn about the properties, reactions. Why Is Iron Reactive.
From revisechemistry.uk
Obtaining and Using Metals Edexcel T4 revisechemistry.uk Why Is Iron Reactive See the full reactivity series, the atomic structure of iron, and the formation and oxidation of iron. Once the sacrificial metal has corroded away, it can simply be replaced. Iron has more potential to form bonds with. Pure iron is quite reactive. It combines vigorously with chlorine on mild heating and. Find out how iron (iii) hydroxide is formed and.. Why Is Iron Reactive.
From www.youtube.com
Trick to remember Reactions of Rusting of iron/Electrochemistry/ASN Why Is Iron Reactive The lower atomic number gives the iron a higher electron affinity than zinc, which makes it more reactive than its counterpart. Learn about the properties, reactions and uses of iron and its compounds. 58 rows learn about the reactivity series of metals, an empirical and analytical progression of their reactivity with water, acids and other. It combines vigorously with chlorine. Why Is Iron Reactive.
From byjus.com
Why does the color of copper sulphate change when an iron nail is kept Why Is Iron Reactive Once the sacrificial metal has corroded away, it can simply be replaced. The more reactive metal oxidises more readily than iron, so it ‘sacrifices’ itself while the iron does not rust. Iron dissolves in water, and reacts. Learn why iron is not very reactive, but compared to other common metals, it is. Find out how iron (iii) hydroxide is formed. Why Is Iron Reactive.
From www.youtube.com
Copper sulphate and iron reaction Experiments Displacement reaction Why Is Iron Reactive Once the sacrificial metal has corroded away, it can simply be replaced. It combines vigorously with chlorine on mild heating and. Learn about the properties, reactions and uses of iron and its compounds. In fact, free iron is not readily found in nature. 58 rows learn about the reactivity series of metals, an empirical and analytical progression of their reactivity. Why Is Iron Reactive.
From www.teachoo.com
Write equations for reaction of (i) iron with steam Class 10 Science Why Is Iron Reactive Iron has more potential to form bonds with. Find out how iron (iii) hydroxide is formed and. It combines vigorously with chlorine on mild heating and. The lower atomic number gives the iron a higher electron affinity than zinc, which makes it more reactive than its counterpart. Pure iron is quite reactive. The element iron is a very chemically reactive. Why Is Iron Reactive.
From www.teachoo.com
Reaction of Metals and Nonmetals with Oxygen Concepts Why Is Iron Reactive Learn about the properties, reactions and uses of iron and its compounds. In fact, free iron is not readily found in nature. Learn why iron is not very reactive, but compared to other common metals, it is. Iron has more potential to form bonds with. Pure iron is quite reactive. The element iron is a very chemically reactive metal, and. Why Is Iron Reactive.
From blog.thepipingmart.com
Which metal is more reactive iron or zinc Why Is Iron Reactive The element iron is a very chemically reactive metal, and reacts openly with oxygen in humid air; Once the sacrificial metal has corroded away, it can simply be replaced. See the full reactivity series, the atomic structure of iron, and the formation and oxidation of iron. 58 rows learn about the reactivity series of metals, an empirical and analytical progression. Why Is Iron Reactive.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) Redox Why Is Iron Reactive Pure iron is quite reactive. Learn about the properties, reactions and uses of iron and its compounds. See the full reactivity series, the atomic structure of iron, and the formation and oxidation of iron. It combines vigorously with chlorine on mild heating and. Once the sacrificial metal has corroded away, it can simply be replaced. Iron has more potential to. Why Is Iron Reactive.
From byjus.com
Why is iron not very reactive? Why Is Iron Reactive The element iron is a very chemically reactive metal, and reacts openly with oxygen in humid air; The lower atomic number gives the iron a higher electron affinity than zinc, which makes it more reactive than its counterpart. Once the sacrificial metal has corroded away, it can simply be replaced. In a very finely divided state metallic iron is pyrophoric. Why Is Iron Reactive.
From www.yaclass.in
Environmental effects of a chemical reaction Rusting — lesson. Science Why Is Iron Reactive Iron has more potential to form bonds with. The element iron is a very chemically reactive metal, and reacts openly with oxygen in humid air; Learn why iron is not very reactive, but compared to other common metals, it is. Learn about the properties, reactions and uses of iron and its compounds. The more reactive metal oxidises more readily than. Why Is Iron Reactive.
From www.teachoo.com
Displacement Reaction and Reactivity Series Concepts Why Is Iron Reactive Learn why iron is not very reactive, but compared to other common metals, it is. Learn about the properties, reactions and uses of iron and its compounds. 58 rows learn about the reactivity series of metals, an empirical and analytical progression of their reactivity with water, acids and other. In a very finely divided state metallic iron is pyrophoric (i.e.,. Why Is Iron Reactive.
From nafarahaza.panel-laboralcj.gob.mx
Reactivity Series Reactivity of Metals Chart, Features, Tricks Why Is Iron Reactive Learn why iron is not very reactive, but compared to other common metals, it is. It combines vigorously with chlorine on mild heating and. Once the sacrificial metal has corroded away, it can simply be replaced. See the full reactivity series, the atomic structure of iron, and the formation and oxidation of iron. In fact, free iron is not readily. Why Is Iron Reactive.
From www.researchgate.net
Role of iron in formation of reactive oxygen species (ROS) Download Why Is Iron Reactive In fact, free iron is not readily found in nature. Learn why iron is not very reactive, but compared to other common metals, it is. It combines vigorously with chlorine on mild heating and. 58 rows learn about the reactivity series of metals, an empirical and analytical progression of their reactivity with water, acids and other. See the full reactivity. Why Is Iron Reactive.
From sciencenotes.org
Activity Series of Metals (Reactivity Series) Why Is Iron Reactive Learn why iron is not very reactive, but compared to other common metals, it is. The element iron is a very chemically reactive metal, and reacts openly with oxygen in humid air; The more reactive metal oxidises more readily than iron, so it ‘sacrifices’ itself while the iron does not rust. In a very finely divided state metallic iron is. Why Is Iron Reactive.
From mungfali.com
The Reactivity Series Of Metals Why Is Iron Reactive The element iron is a very chemically reactive metal, and reacts openly with oxygen in humid air; In a very finely divided state metallic iron is pyrophoric (i.e., it ignites spontaneously). Iron has more potential to form bonds with. The lower atomic number gives the iron a higher electron affinity than zinc, which makes it more reactive than its counterpart.. Why Is Iron Reactive.
From scienceislife4sgss.weebly.com
Metals and NonMetals SCIENCE IS LIFE Why Is Iron Reactive It combines vigorously with chlorine on mild heating and. The element iron is a very chemically reactive metal, and reacts openly with oxygen in humid air; The more reactive metal oxidises more readily than iron, so it ‘sacrifices’ itself while the iron does not rust. In fact, free iron is not readily found in nature. See the full reactivity series,. Why Is Iron Reactive.
From www.myvitacine.com
Iron Overload and Oxidation Vitacine Why Is Iron Reactive Learn about the properties, reactions and uses of iron and its compounds. Pure iron is quite reactive. See the full reactivity series, the atomic structure of iron, and the formation and oxidation of iron. 58 rows learn about the reactivity series of metals, an empirical and analytical progression of their reactivity with water, acids and other. Learn why iron is. Why Is Iron Reactive.
From www.dreamstime.com
The Chemical Required for Rust Formation Vector Illustration Stock Why Is Iron Reactive 58 rows learn about the reactivity series of metals, an empirical and analytical progression of their reactivity with water, acids and other. It combines vigorously with chlorine on mild heating and. In a very finely divided state metallic iron is pyrophoric (i.e., it ignites spontaneously). The lower atomic number gives the iron a higher electron affinity than zinc, which makes. Why Is Iron Reactive.
From www.dyna-nutrition.com
Why iron matters? Dynamic Nutrition Why Is Iron Reactive Learn about the properties, reactions and uses of iron and its compounds. See the full reactivity series, the atomic structure of iron, and the formation and oxidation of iron. Iron dissolves in water, and reacts. Pure iron is quite reactive. It combines vigorously with chlorine on mild heating and. 58 rows learn about the reactivity series of metals, an empirical. Why Is Iron Reactive.
From www.thesciencehive.co.uk
The Reactivity Series* — the science sauce Why Is Iron Reactive It combines vigorously with chlorine on mild heating and. Once the sacrificial metal has corroded away, it can simply be replaced. Learn about the properties, reactions and uses of iron and its compounds. In a very finely divided state metallic iron is pyrophoric (i.e., it ignites spontaneously). The element iron is a very chemically reactive metal, and reacts openly with. Why Is Iron Reactive.