Catalyst Inhibitor Definition . Describe the similarities and differences between the three principal classes of catalysts. It may also be called a negative catalyst. In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. In chemistry, an inhibitor is a substance that delays, slows or prevents a chemical reaction. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. They work by interfering with the. The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? In heterogeneous catalysis, catalysts provide a surface to which.
from www.worksheetsplanet.com
In heterogeneous catalysis, catalysts provide a surface to which. What are catalysts, and how do they work in terms altering the parameters of a reaction? They work by interfering with the. In chemistry, an inhibitor is a substance that delays, slows or prevents a chemical reaction. It may also be called a negative catalyst. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Describe the similarities and differences between the three principal classes of catalysts. In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures.
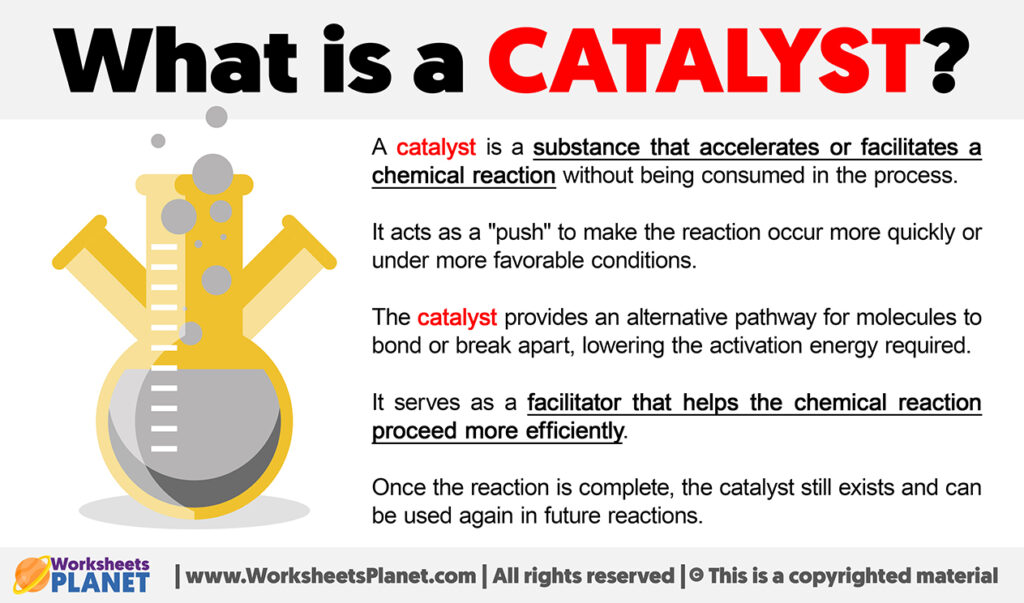
What is a Catalyst Definition of Catalyst
Catalyst Inhibitor Definition In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. Describe the similarities and differences between the three principal classes of catalysts. In chemistry, an inhibitor is a substance that delays, slows or prevents a chemical reaction. In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. They work by interfering with the. In heterogeneous catalysis, catalysts provide a surface to which. The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. It may also be called a negative catalyst. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction.
From www.tebubio.com
Why are certain drugs called enzyme inhibitors? Catalyst Inhibitor Definition The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. Describe the similarities and differences between the three principal classes of catalysts. What are catalysts, and how do they work in terms altering the parameters of a reaction? It may also be called a negative catalyst.. Catalyst Inhibitor Definition.
From ceirupig.blob.core.windows.net
Enzymes Low Temp at Gilbert Johnson blog Catalyst Inhibitor Definition In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. Describe the similarities and differences between the three principal classes of catalysts. The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. Catalysts allow a reaction to proceed via a pathway. Catalyst Inhibitor Definition.
From bio1151.nicerweb.com
catalytic.html 08_17CatalyticCycle.jpg Catalyst Inhibitor Definition Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. They work by interfering with the. Describe the similarities and differences between the three principal classes of catalysts. In chemistry, an inhibitor is a substance that delays, slows or prevents a chemical reaction. It may also be. Catalyst Inhibitor Definition.
From www.biologyexams4u.com
Reversible Enzyme Inhibition Competitive, Non Competitive and Catalyst Inhibitor Definition They work by interfering with the. The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. It may also be called a negative catalyst. Describe the similarities and differences between the three principal classes of catalysts. Catalysts allow a reaction to proceed via a pathway that. Catalyst Inhibitor Definition.
From loemotkul.blob.core.windows.net
What Is A Catalyst In A Chemical Reaction at Richard Starr blog Catalyst Inhibitor Definition The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. They work by interfering with the. What are catalysts, and how do they work in terms altering the parameters of a reaction? In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical. Catalyst Inhibitor Definition.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalyst Inhibitor Definition In heterogeneous catalysis, catalysts provide a surface to which. What are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences between the three principal classes of catalysts. In chemistry, an inhibitor is a substance that delays, slows or prevents a chemical reaction. Catalysts allow a reaction to proceed via a. Catalyst Inhibitor Definition.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID Catalyst Inhibitor Definition It may also be called a negative catalyst. In chemistry, an inhibitor is a substance that delays, slows or prevents a chemical reaction. The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. In heterogeneous catalysis, catalysts provide a surface to which. Describe the similarities and. Catalyst Inhibitor Definition.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation ID591293 Catalyst Inhibitor Definition Describe the similarities and differences between the three principal classes of catalysts. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. In contrast to catalysts, inhibitors are substances that decrease. Catalyst Inhibitor Definition.
From www.slideserve.com
PPT Nanocatalyst PowerPoint Presentation, free download ID676158 Catalyst Inhibitor Definition In heterogeneous catalysis, catalysts provide a surface to which. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of. Catalyst Inhibitor Definition.
From joivzdnat.blob.core.windows.net
Enzyme Biochemistry Britannica at Joleen Stanfield blog Catalyst Inhibitor Definition In chemistry, an inhibitor is a substance that delays, slows or prevents a chemical reaction. It may also be called a negative catalyst. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. They work by interfering with the. In heterogeneous catalysis, catalysts provide a surface to. Catalyst Inhibitor Definition.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalyst Inhibitor Definition Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to which. In chemistry, an inhibitor is a substance that delays, slows or prevents a chemical reaction. In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. What are. Catalyst Inhibitor Definition.
From www.youtube.com
Phase Transfer Catalyst (PTC) Chemistry by Dr. Tanmoy Biswas Catalyst Inhibitor Definition The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. It may also be called a negative catalyst. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. In contrast to catalysts, inhibitors. Catalyst Inhibitor Definition.
From teachmephysiology.com
Enzyme Inhibition Types of Inhibition TeachMePhysiology Catalyst Inhibitor Definition In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? In chemistry, an inhibitor is a substance that delays, slows or prevents a chemical reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation. Catalyst Inhibitor Definition.
From www.differencebetween.com
Difference Between Catalyst and Inhibitor Compare the Difference Catalyst Inhibitor Definition Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Describe the similarities and differences between the three principal classes of catalysts. In heterogeneous catalysis, catalysts provide a surface to which. They work by interfering with the. In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical. Catalyst Inhibitor Definition.
From www.jeremyleslie.co.nz
Are you a catalyst or an inhibitor? — Jeremy Leslie Catalyst Inhibitor Definition It may also be called a negative catalyst. In heterogeneous catalysis, catalysts provide a surface to which. They work by interfering with the. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. What are catalysts, and how do they work in terms altering the parameters of. Catalyst Inhibitor Definition.
From differencebtw.com
Catalyst vs. Inhibitor Know the Difference Catalyst Inhibitor Definition The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. They work by interfering with the. Describe the similarities and differences between the. Catalyst Inhibitor Definition.
From www.askdifference.com
Catalyst vs. Inhibitor — What’s the Difference? Catalyst Inhibitor Definition In chemistry, an inhibitor is a substance that delays, slows or prevents a chemical reaction. The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. In heterogeneous catalysis, catalysts provide a surface to which. In contrast to catalysts, inhibitors are substances that decrease the rate of. Catalyst Inhibitor Definition.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID Catalyst Inhibitor Definition What are catalysts, and how do they work in terms altering the parameters of a reaction? In chemistry, an inhibitor is a substance that delays, slows or prevents a chemical reaction. In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot. Catalyst Inhibitor Definition.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst Inhibitor Definition In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. Describe the similarities and differences between the three principal classes of catalysts. In heterogeneous catalysis, catalysts provide a surface to which. The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures.. Catalyst Inhibitor Definition.
From www.dreamstime.com
Catalyst Competitive Inhibition Active Site Stock Vector Illustration Catalyst Inhibitor Definition It may also be called a negative catalyst. In heterogeneous catalysis, catalysts provide a surface to which. In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. Describe the similarities and differences between the three principal classes of catalysts. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than. Catalyst Inhibitor Definition.
From pediaa.com
What is the Difference Between Competitive and Catalyst Inhibitor Definition They work by interfering with the. The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. Describe the similarities and differences between the three principal classes of catalysts. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance. Catalyst Inhibitor Definition.
From www.sqadia.com
Enzyme Inhibitors Catalyst Inhibitor Definition The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. In chemistry, an inhibitor is a substance that delays, slows or prevents a chemical reaction. In heterogeneous catalysis, catalysts provide a surface to which. What are catalysts, and how do they work in terms altering the. Catalyst Inhibitor Definition.
From www.slideserve.com
PPT Nanocatalyst PowerPoint Presentation, free download ID1563031 Catalyst Inhibitor Definition In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. In heterogeneous catalysis, catalysts provide a surface to which. In chemistry, an inhibitor is a substance that delays, slows or prevents a chemical reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis, the. Catalyst Inhibitor Definition.
From www.numerade.com
SOLVED Consider the following reaction mechanism Step 1 ICl + H2 Catalyst Inhibitor Definition In chemistry, an inhibitor is a substance that delays, slows or prevents a chemical reaction. In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. They work by interfering with the. The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures.. Catalyst Inhibitor Definition.
From www.dubuy.com
Enzyme Dictionary Instant notes on definition, catalysis, mechanism Catalyst Inhibitor Definition The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. It may also be called a negative catalyst. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysts allow a reaction to. Catalyst Inhibitor Definition.
From www.researchgate.net
Equilibrium in the system "Karstedt's catalystinhibitor.". Download Catalyst Inhibitor Definition It may also be called a negative catalyst. Describe the similarities and differences between the three principal classes of catalysts. In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. They work by interfering with the. In heterogeneous catalysis, catalysts provide a surface to which. What are catalysts, and how do they work in terms. Catalyst Inhibitor Definition.
From exoyjbtvu.blob.core.windows.net
What Are Enzyme Inhibitors Quizlet at Norma Franklin blog Catalyst Inhibitor Definition In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. They work by interfering with the. In heterogeneous catalysis, catalysts provide a surface to which. Describe the similarities and differences between the three principal classes of catalysts. The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1. Catalyst Inhibitor Definition.
From ablogwithadifference.com
Catalyst and Inhibitor The best 5 difference Catalyst Inhibitor Definition It may also be called a negative catalyst. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. In heterogeneous catalysis, catalysts provide a surface to which. Describe the similarities and differences between the three principal classes of catalysts. In chemistry, an inhibitor is a substance that. Catalyst Inhibitor Definition.
From pediaa.com
What is the Difference Between Enzyme Activator and Enzyme Inhibitor Catalyst Inhibitor Definition Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. In heterogeneous catalysis, catalysts provide a surface to which. They work by interfering with the. The catalyst/inhibitor system and their respective concentrations are selected to give. Catalyst Inhibitor Definition.
From www.mdpi.com
Catalysts Free FullText Is ATP the Only Nucleoside Triphosphate Catalyst Inhibitor Definition Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. They work by interfering with the. In contrast to catalysts, inhibitors are substances that decrease the rate of. Catalyst Inhibitor Definition.
From www.worksheetsplanet.com
What is a Catalyst Definition of Catalyst Catalyst Inhibitor Definition They work by interfering with the. Describe the similarities and differences between the three principal classes of catalysts. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction.. Catalyst Inhibitor Definition.
From www.youtube.com
Catalyst and Inhibitors YouTube Catalyst Inhibitor Definition The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. It may also be called a negative catalyst. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In contrast to catalysts, inhibitors are substances that decrease. Catalyst Inhibitor Definition.
From joilxpqmt.blob.core.windows.net
Chemical Catalysis Examples at Howard Wade blog Catalyst Inhibitor Definition They work by interfering with the. The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysts allow a reaction to proceed via. Catalyst Inhibitor Definition.
From www.mdpi.com
Catalysts Free FullText Peroxymonosulfate Activation by CuOFe2O3 Catalyst Inhibitor Definition Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. It may also be called a negative catalyst. Describe the similarities and differences between the three principal classes of catalysts. In heterogeneous catalysis, catalysts provide a surface to which. In contrast to catalysts, inhibitors are substances that decrease the rate of. Catalyst Inhibitor Definition.
From joicbgfdr.blob.core.windows.net
Catalyst Definition Speech at Ashley Depriest blog Catalyst Inhibitor Definition Describe the similarities and differences between the three principal classes of catalysts. What are catalysts, and how do they work in terms altering the parameters of a reaction? The catalyst/inhibitor system and their respective concentrations are selected to give a typical pot life ranging from 1 to 8 h at ambient temperatures. It may also be called a negative catalyst.. Catalyst Inhibitor Definition.